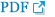|
 |
- Search
| Precis Future Med > Volume 3(1); 2019 > Article |
|
Abstract
Purpose
The Adjuvant chemoRadioTherapy In Stomach Tumors 2 (ARTIST 2) trial was conducted to compare the efficacy between adjuvant chemotherapy regimens and chemoradiotherapy in D2-resected, node-positive, stage 2 or 3 gastric cancer.
Methods
In this prospective, multicenter, phase IIItrial, we randomly assigned patients to three treatment arms: patients who receive adjuvant S-1 for 1 year, S-1 plus oxaliplatin (SOX) for 6 months, or SOX plus chemoradiotherapy (SOXRT). Herein, we report the safety outcomes of patients who received adjuvant chemotherapy or chemoradiotherapy.
Results
Among a total of 514 patients registered between February 2013 and December 2017, 499 patients who either completed or discontinued the assigned study treatments were included in the present analysis. Allthe three treatment arms were generally well-tolerated, with the overall treatment completion rate of 94% (96% in S-1, 93% in SOX, and 92% in SOXRT). The median delivered dose of radiotherapy in the SOXRT arm was 4,500 cGy (range, 0 to 4,500 cGy). The most frequently observed adverse events were fatigue (29%) in S-1 arm and peripheral neuropathy in the SOX and SOXRT arms (59% and 50%,respectively).
Although complete resection with regional lymph node dissection (LND) is the only curative treatment for patients with gastric cancer (GC), a significant percentage of patients undergoing curative surgery will experience recurrence, leading to a poor prognosis. It has been more than a decade ago since the results from the Intergroup-0116 trial [1] showed that adjuvant chemoradiotherapy significantly improved overall survival (OS) and established radiotherapy as part of the standard of care for the adjuvant treatment of GC. The role of adding radiotherapy to adjuvant chemotherapy in Asian countries, where gastrectomy plus D2 LND followed by adjuvant chemotherapy has already been the standard of care [2,3], has been brought into question, and we failed to demonstrate a difference in disease-free survival (DFS) in the Adjuvant chemoRadioTherapy In Stomach Tumors (ARTIST) trial [4,5]. Although the ARTIST trial was a negative trial, we observed that improved outcomes could be achieved by adding radiotherapy in certain patient subsets [5,6], including those with node-positive disease or higher lymph node (LN) ratio and intestinal-type GC.
The ARTIST 2 trial was conducted to compare the efficacy between adjuvant chemotherapy regimens and chemoradiotherapy in D2-resected, LN-positive, stage 2 or 3 GC. The purpose of the present interim report was to evaluate the safety of the three study treatment regimens, in part justifying the continuation of the trial.
The ARTIST 2 trial (ClinicalTrials.gov, NCT0176146) was conducted in accordance with the ethical principles of the Declaration of Helsinki and local guidelines. The Institutional Review Boards (SMC IRB 2012-06-061) or ethics committees of all participating centers reviewed and approved the protocol. The trial was registered at ClinicalTrials.gov (NCT0176146). All patients provided written informed consent after having been informed about the purpose and investigational nature of the study.
Patients were eligible in this multicenter, open-label, randomized phase III Korean trial if they had histologically confirmed adenocarcinoma of the stomach; had undergone complete surgical resection of the primary tumor without residual disease and D2 LND, pathologically staged 2 or 3 according to the 7th edition of the American Joint Committee on Cancer (AJCC 2010) system; had LN-positive disease (i.e., N1, N2, or N3), had an age of 20 years or older, had a score of 0 or 1 based on the Eastern Cooperative Oncology Group performance status; and had adequate major organ functions. Patients with coexisting malignancies or who were unable to tolerate chemotherapy because of other systemic illnesses or difficulty in swallowing oral medications were excluded from the study. Patients who had undergone gastrectomy, with D2 LND less likely considered, were also excluded.
Eligible patients were stratified according to pathologic stage (2 or 3), type of surgery (total or subtotal gastrectomy), and the Lauren classification (intestinal, diffuse, or mixed/unknown). They were then randomly assigned to receive adjuvant S-1 (given orally twice daily on days 1 to 28, at a dose of 80 mg/day for patients with a body surface area [BSA] < 1.25 m2, 100 mg/day for patients with a BSA of 1.25 m2 to < 1.5 m2, and 120 mg/day for patients with a BSA of ≥ 1.5 m2) every 6 weeks for up to eight cycles, S-1 plus oxaliplatin (SOX; S-1 twice daily on days 1 to 14 plus oxaliplatin 130 mg/m2 intravenously on day 1) every 3 weeks for up to eight cycles, or SOX plus chemoradiotherapy (SOXRT; 2 cycles of SOX and then 5 weeks of radiotherapy with continuous S-1, 40 mg twice daily, during radiotherapy, followed by four additional cycles of SOX) (Fig. 1). Details of the concurrent chemoradiotherapy involving S-1 have been described previously [7]. As a summary, three-dimensional conformal radiotherapy target included the tumor bed in T4 disease or the regional LN area, anastomosis site, and duodenal stump if the surgical resection margins were less than 3 cm. Radiotherapy was performed using 10-megavolt photon beams to 45 Gy with 1.8 Gy daily fractions administered five times a week.
Dose reductions and/or administration delays are provided in case of severe hematological or non-hematological toxicities while on study treatment. Dose adjustments were made according to the system showing the greatest degree of toxicity using the Common Terminology Criteria for Adverse Events (CTCAE v4.0). Chemotherapy was delayed if grade 2 or higher hematologic toxicities were observed. If hematologic toxicities lowered after a 1-week delay, chemotherapy was performed without dose reductions. If a patient required a delay of longer than 3 weeks for recovery, treatment was discontinued, and the patient was treated outside the clinical trial. For grade 2 or higher non-hematologic toxicities, chemotherapy was delayed until the patient’s symptoms were reduced to grade 1 or less. In cases of grade 2 hand-foot syndrome and peripheral neuropathy, the doses of S-1 and oxaliplatin, respectively, were reduced. Investigators were free to provide non-protocol supportive care measures any time during the study if it was for the patient’s best interest.
All patients adhered to the same schedule of follow-up visits during and after the completion of study treatment, which required recording of symptoms, toxicities, and laboratory and imaging studies. Abdominal computed tomography scans and upper gastrointestinal endoscopy were scheduled to occur every 3 months during study treatment, and every 6 months after completion, up to 5 years.
The primary endpoint of the ARTIST 2 trial was DFS, and secondary endpoints included OS and safety. DFS was measured from the date of surgery until death, relapse, or presence of second primary tumor, whichever occurred first. In the present randomized trial, the S-1 arm was designated as the control arm. Based on previous study [3], adjuvant S-1 would provide a 3-year DFS rate of 72% and an annual hazard rate of recurrence of 0.11. To test if experimental arms (SOX or SOXRT) lower the hazard rate by 50% (i.e., hazard ratio of 1.5), at least 855 eligible patients (or 300 patients per arm assuming 5% attrition due to ineligibility or dropout) were needed to have a 90% of overall power (i.e., 3-year DFS rate is 72% for S-1 arm and 80.33% for any the two experimental arms). Interim analyses for safety and efficacy, as well as the Independent Data Monitoring Committee (IDMC), were planned to be done every year, and the present report contains the safety outcomes described at the end of 2017. No formal comparisons of toxicities between treatment arms are provided.
Between February 2013 and December 2017, a total of 514 patients (57% of the planned sample size) were entered onto the ARTIST 2 trial. Among them, 499 patients who either completed or discontinued the assigned study treatments were included in the present interim analysis. All patients were randomized: 152 patients to the S-1 arm, 147 to the SOX arm, and 150 to the SOXRT arm. Baseline patient and tumor characteristics were generally well balanced across treatment arms (Table 1). Men comprised two-thirds of the patients, and the median age of the patients was 66 years (range, 28 to 82 years). Overall, 305 patients (61%) had received subtotal gastrectomy, 241 (48%) had diffuse-type histology, and 290 (58%) had a stage 3 disease.
All the three treatment arms were generally well-tolerated, with the overall treatment completion rate of 94% (96% in arm A, 93% in arm B, and 92% in arm C, respectively). The median delivered dose of radiotherapy in arm C was 4,500 cGy (range, 0 to 4,500 cGy). Compliance to study treatment showed no difference according to the type of surgery or stage. The following were the reasons for the premature discontinuation during the study treatment: patient withdrawal (n = 33), occurrence of adverse events (n = 25), recurrence (n= 23), and protocol violation (n= 4). During the study treatment, surgery-related complications were rarely observed: wound dehiscence in four patients (three in the SOX arm, one in the S-1 arm), adhesive ileus in three patients (one in each arm), and pyloric stenosis in two patients (2 in the S-1 arm). The most frequently observed adverse events (Table 2) in arms A, B, and C were fatigue (29%) in arm A, and peripheral neuropathy in arms B and C (59% and 50%, respectively). Most adverse events were grade 1 or 2 and thus considered easily manageable. Two patients died during the study treatment. A 68-year-old male patient (S-1 arm) died of sudden cardiac arrest shortly after the first cycle of S-1. Although his baseline electrocardiography, as well as the preoperative cardiac function, was completely normal, the possibility of drug-related mortality was not completely excluded. Another patient, a 49-year-old male (SOXRT arm), died of an in-car accident during the first month of the study treatment.
From 2014, we did interim analyses of safety at the end of each year, and the present report contained the results from the 4th interim analysis. At the end of 2017, the IDMC was held to allow study termination if treatment tolerability was unacceptable. After a discussion within the IDMC, the stopping rules were not activated, allowing the continuation of the recruitment.
As discussed earlier [4,5], our ARTIST trial confirmed that the patients with D2-resected GC can achieve long-term DFS with both adjuvant chemotherapy and chemoradiotherapy. The ARTIST trial [5], together with the results from other randomized trials comparing adjuvant chemotherapy with observation [2,3], provided additional support for adjuvant chemotherapy with either S-1 for 1 year or fluoropyrimidine plus oxaliplatin for 6 months as standards of care in those patients. Although we failed to observe significant difference in DFS between adjuvant chemoradiotherapy and chemotherapy, there may be a subset of patients who may benefit from the addition of radiotherapy to adjuvant chemotherapy (i.e., LN-positive disease). Based on the observation, and since the optimal adjuvant regimen has yet to be identified, we designed the present ARTIST 2 trial comparing adjuvant S-1, SOX, and SOXRT in LN-positive, pathologically staged 2 or 3, D2-resected GC within the framework of clinical trials.
There remains controversy surrounding the choice of adjuvant therapy for D2-resected GC. After the publication of ACTS-GC trial [3], adjuvant S-1 for 1 year has become one of the standard treatments in patients treated with curative D2 gastrectomy for stage 2 or 3 GC. However, the 5-year OS rates in stage 3a and stage 3b patients receiving adjuvant S-1 were 67% and 50%, respectively, which were less satisfactory compared with the rate for stage 2 patients (84%) [8]. In the CLASSIC trial comparing adjuvant capecitabine plus oxaliplatin with surgery alone [9], the 5-year OS rate for stage 2 GC fared significantly better compared with the rate for stage 3a and stage 3b disease (70% and 66%, respectively). These trials led to different treatment strategies to GC patients. While single-agent S-1 for 1 year now seems likely to be the recommended adjuvant treatment for the stage 2 GC patients, combination chemotherapy involving oxaliplatin has become the most widely administered adjuvant treatment for the stage 3 disease [10]. Even in Japan, where S-1 is the only available adjuvant therapy for GC, SOX is commonly administered for patients with stage 3 GC [11]. On the contrary, in Western countries, where D2 LND is less likely considered as the standard surgery for GC, chemoradiotherapy is the most frequently recommended adjuvant treatment [5].
Our preliminary report confirms that the three treatment arms in the ARTIST 2 trial were generally well-tolerated. The frequencies of adverse events in the three treatment arms were similar to those already reported in adjuvant trials. Mild-to- moderate fatigue was the most frequently observed adverse event in the S-1 arm, and, as expected, peripheral neuropathy was frequently observed in the SOX and SOXRT arms. There has been some speculation about oxaliplatin-related peripheral neuropathy. The incidence of peripheral neuropathy is known to be associated with the prolonged use of oxaliplatin [12]. However, most cases were limited to grade 1 or 2, and it should be noted that the possibility of developing severe oxaliplatin neuropathy was not related to the cumulative dose or treatment duration [13]. In support of our belief that the S-1, SOX, and SOXRT arms are feasible in the adjuvant setting, we observed very few ( < 5%) patients who discontinued the study treatment due to the occurrence of adverse events.
In conclusion, we found no significant safety concerns in the ARTIST 2 trial. According to the IDMC, patient accrual is underway. Issues raised by the ARTIST and other randomized trials in the adjuvant setting of GC should hopefully be answered by the ARTIST 2 trial, with the following hypothesis: the addition of oxaliplatin to S-1 and the addition of radiotherapy to adjuvant chemotherapy will improve DFS. Compared to the previous ARTIST trial [4], this trial was designed as a multicenter study in order to increase patient recruitment. However, due to the eligibility criteria involving only patients with LN-positive, stage 2 or 3 GC, enrollment is a bit slower than expected.
ACKNOWLEDGEMENTS
This work was supported by Samsung Medical Center, Seoul, South Korea (Grant Number: SMO-1131311).
Fig. 1.
Study diagram for the Adjuvant chemoRadioTherapy In Stomach Tumors 2 (ARTIST 2) trial. STG, subtotal gastrectomy; TG, total gastrectomy; SOX, S-1 plus oxaliplatin; SOXRT, SOX plus chemoradiotherapy; RT, radiotherapy; bid, two times a day.
Table 1.
Baseline patient and tumor characteristics
| Characteristic | S-1 | SOX | SOXRT |
|---|---|---|---|
| No. of patients | 152 | 147 | 150 |
| Age (yr) | |||
| Median | 67 | 66 | 66 |
| Range | 32–82 | 28–77 | 29–74 |
| Sex | |||
| Male | 101 | 99 | 99 |
| Female | 51 | 48 | 51 |
| Type of gastrectomy | |||
| Total | 50 | 40 | 54 |
| Subtotal | 102 | 107 | 96 |
| Lauren classification | |||
| Diffuse | 78 | 83 | 80 |
| Intestinal | 50 | 52 | 46 |
| Mixed/unknown | 24 | 12 | 24 |
| Performance status | |||
| 0 | 76 | 82 | 84 |
| 1 | 76 | 65 | 66 |
| Pathologic stagea) | |||
| 2 | 57 | 51 | 51 |
| 3 | 95 | 96 | 99 |
Table 2.
Maximum grade adverse events
REFERENCES
1. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725–30.


2. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315–21.


3. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810–20.


4. Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268–73.


5. Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol 2015;33:3130–6.


6. Kim Y, Park SH, Kim KM, Choi MG, Lee JH, Sohn TS, et al. The influence of metastatic lymph node ratio on the treatment outcomes in the adjuvant chemoradiotherapy in stomach tumors (ARTIST) trial: a phase III trial. J Gastric Cancer 2016;16:105–10.



7. Lee SJ, Sohn TS, Lee J, Park SH, Park JO, Lim DH, et al. Adjuvant chemoradiation with 5-fluorouracil/leucovorin versus S-1 in gastric cancer patients following D2 lymph node dissection surgery: a feasibility study. Anticancer Res 2014;34:6585–91.

8. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387–93.


9. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389–96.


10. Lee HY, Hwang IG, Park SE, Kim MJ, Park SH, Kang JH, et al. Factors influencing clinicians’ choice of adjuvant S-1 versus capecitabine plus oxaliplatin after curative gastrectomy in patients with gastric cancer. J Cancer 2016;7:1711–5.



11. Shitara K, Chin K, Yoshikawa T, Katai H, Terashima M, Ito S, et al. Phase II study of adjuvant chemotherapy of S-1 plus oxaliplatin for patients with stage III gastric cancer after D2 gastrectomy. Gastric Cancer 2017;20:175–81.