 |
 |
- Search
| Precis Future Med > Volume 4(3); 2020 > Article |
|
Abstract
Infectious diseases and stroke are common and a great health problem worldwide. However, relatively few studies have focused on direct infectious causes of acute stroke, and the exact mechanisms of acute stroke associated with infections are poorly understood. Herein, we present several infectious organisms that are probable causes of stroke. Different pathomechanisms of stroke and neuroimaging features of infection by these organisms are discussed. Specifically, the distribution of cerebral infarction suggests that infections could be a direct cause of acute stroke. These include cerebral convexity probably due to vasculitis in bacterial meningitis, deep infarcts due to endarteritis and inflammation in neurocysticercosis, and exudates in tuberculous meningitis, as well as hemodynamic borderzone infarct due to large vessel vasculitis by direct invasion of fungal infection. We suggest that prompt and appropriate control of these organisms could prevent ischemic stroke.
Infectious diseases remain a great health problem in developing countries and the proportion of infection-related strokes is possibly greater in these nations [1]. Furthermore, over the last decades, the number of immunocompromised patients has increased in part due to the use of immunosuppressive treatments (e.g., post-organ transplantation) and human immunodeficiency virus (HIV) infection.
Some infectious pathogens may act as risk factors and can trigger stroke through different mechanisms in their acute phase. Most studies to date have focused on the role of chronic infections/inflammation as a risk or trigger factor for stroke and atherosclerosis, or on the control of post-stroke infection [2-6]. Relatively, fewer studies have focused on direct infectious causes of acute stroke [7,8]. Consequently, the exact mechanisms underlying acute stroke associated with infection are poorly understood.
Here, we introduce several cases with various intracranial infections that caused acute ischemic stroke. Different pathomechanisms of stroke and different neuroimaging features associated with these organisms are discussed.
A 62-year-old man developed general weakness and a fever/chilling sensation. He was taking prednisolone and azathioprine due to immunoglobulin G4-related sclerosing cholangitis and pancreatitis. Twelve days after the onset of fever, he developed mental changes and speech disturbance. Cerebrospinal fluid (CSF) examination showed elevated intracranial pressure (> 30 cmH2O) and white blood cell (WBC) count 38,400/µL (polymorphonuclear neutrophils [PMNs] 82%, lymphocytes 3%). Brain magnetic resonance imaging (MRI) showed meningeal enhancement and fluid collection. Based on the CSF study and MRI findings, he was diagnosed as having bacterial meningitis, and underwent external ventricular drainage. Antibiotic and steroid therapy were started. CSF culture revealed Klebsiella pneumonia. The fever subsided, but the patient remained severely disabled (Fig. 1).
A 70-year-old woman developed swelling in her left ocular region followed by ocular pain, left ptosis, and diplopia 2 days later. She had diabetes and hypertension. Two weeks later, right hemiparesis and aphasia developed. A physical examination revealed no fever, and black necrotic mucosal eschar and mucosal ulceration were observed in the nasal cavity. A neurological examination showed decreased left visual acuity (light perception), complete ophthalmoplegia, and mild (grade 4+) right hemiparesis and global aphasia. CSF pressure was normal, but WBC was 25/µL (PMNs 43% and lymphocytes 36%) and protein level was 57.1 mg/dL. MRI showed acute infarct on the left borderzone area and occlusion of the previously intact left distal internal carotid artery with wall enhancement. Cranial MRI showed infiltrative enhancing lesions involving the left cavernous sinus, orbital apex, and optic nerve, suggestive of invasive fungal sinusitis. A nasal cavity biopsy from the right middle turbinate was performed, which revealed invasive aspergillosis. The patient received antiplatelet agents, voriconazole, an antifungal agent, and hydration. Infarct growth was observed within the borderzone area, and the patient continued to present with global aphasia and severe right hemiparesis (Fig. 2).
A 48-year-old female presented with fever and headache. On the 2nd day after admission, she developed mental changes and visual hallucinations. CSF examination showed elevated pressure (400 mmH2O) and WBC 120/µL (lymphocytes 66%, monocytes 28%), with significantly elevated protein (224.8 mg/dL) and decreased chloride (114 mmol/L). CSF glucose level was 48.6 mg/dL. Tuberculosis was diagnosed and she was given anti-tuberculosis drugs. One month later, she experienced dizziness and unsteady walking, and brain MRI showed a small infarction in the left medulla. Magnetic resonance angiogram showed normal large vessels. CSF examination at that time showed continued elevated pressure (22 cmH2O) and WBC 90/µL (lymphocytes 70%, monocytes 18%). Another small deep infarct was observed in the right thalamus on the follow-up MRI (Fig. 3).
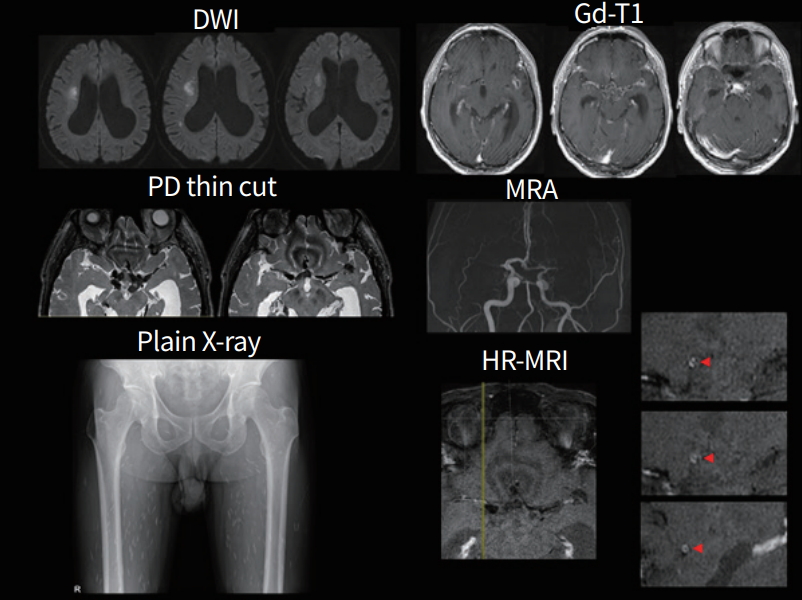
A 59-year-old man experienced transient dysarthria that lasted 30 minutes. He had no history of illness, except hypertension. The neurological examination was unremarkable. MRI showed acute infarcts on the right basal ganglia and corona radiata, and multiple enhancing cystic lesions on the basal cistern. Hydrocephalus and calcified cystic nodules in the cisternal space were also observed. High-resolution MRI showed occlusion of the bilateral proximal middle cerebral arteries (MCAs), as well as circular enhancement and thickening of the MCAs, anterior cerebral arteries, and the distal internal carotid arteries; suggesting vasculitis. CSF showed WBC 8/µL (PMNs 47%, lymphocytes 35%, eosinophils 1%) and protein 77.2 mg/dL. An enzyme-linked immunosorbent assay (ELISA) test for parasite antibodies was positive for cysticercosis. Plain X-ray showed multiple elongated oval shaped, rice grain appearance calcifications within the bilateral leg muscles. Other tests, including malignancy and ring finger protein 213 (RNF213) gene variant for Moyamoya disease, were negative. Albendazole, praziquantel, and prednisolone were started after diagnosis of cysticercosis-related vasculitis. The patient remained functionally independent and free from recurrence (Fig. 4).
Written consent by the patients was waived due to a retrospective nature of our study.
Although anecdotal evidence exists to support a link between most infectious pathogens and stroke, certain pathogens display more robust associations, such that causation is probable [7].
Bacterial meningitis is a serious and life-threatening disease. The occurrence of stroke is associated with worse outcomes in patients with bacterial meningitis. Streptococcus pneumoniae and Neisseria meningitidis are the most common pathogens, although Klebsiella pneumoniae was identified in our case. Infarction can develop weeks after initial diagnosis of meningitis. The arterial involvement in patients with bacterial meningitis is explained by vasospasm of large arteries and vasculitis of small arteries. In our case, proximal large cerebral vessels were spared, and infarcts typically involved the bilateral superficial cortical areas, suggesting that vasculitis involved small MCA branches on the cerebral convexity, rather than large cerebral vessels such as the MCA trunk. Other possible mechanisms include endocarditis/septic emboli and intra-arterial clotting. Because bacterial meningitis may be complicated by intracranial hemorrhage and the role of thrombus is limited in the mechanisms of stroke in patients with bacterial meningitis, the role of antithrombotics is limited and antibiotics and adjunctive steroid therapy are the mainstay of treatment in these patients. However, the prognosis is generally poor and most patients remained functionally dependent in cases with cerebral vascular involvement [9].
Fungal infection is an opportunistic infection and typically occurs in immunocompromised or diabetes patients. Often patients remain afebrile. Mechanisms of stroke differ among fungal pathogens. In cryptococcal infection (most important yeast), meningitis and abscesses are common, and stroke can occur by inflammation or irritation/vasospasm of subarachnoid blood vessels, resulting in small deep infarcts. On the contrary, molds (such as Aspergillus or mucormycosis) rarely cause meningitis, but can directly invade the walls of major intracranial arteries from the nasal cavity and lead to occlusion, causing hemodynamic stroke as presented in this report.
Among parasite infections, neurocysticercosis is the most important human pathogen associated with stroke. Subarachnoid cysticerci produce abnormal thickening of the basal leptomeninges, secondary to an inflammatory exudate. Blood vessels arising from the circle of Willis are usually trapped within this exudate and invaded by inflammatory cells. The end result of this process is endarteritis and subsequent cerebral infarction. In our case, we added steroid therapy in addition to anti-helminthic therapy to prevent a secondary inflammatory reaction caused by the destruction of the cysts which may enhance the process of endarteritis [10].
Tuberculosis remains a serious public health problem, and 25% of the world’s population is infected by Mycobacterium tuberculosis. Infarcts in tuberculosis usually manifest in tuberculous meningitis, and strokes in tuberculosis occur in 15% to 60% of people with tuberculosis [11]. More than half of the patients show normal angiograms, and up to three-fourths of patients show infarcts located in the deep brain regions (‘the tubercular zone’—caudate, thalamus, and internal capsule) [12]. Leptomeningeal exudates surround the brainstem and encase and infiltrate the vessel walls, and may cause endarteritis.
Lastly, viruses have been implicated in increasing the risk of ischemic and hemorrhagic stroke. Herpes zoster infection is the most important viral pathogen associated with stroke. Patients may develop herpes zoster ophthalmicus and contralateral hemiparesis several weeks later. A recent meta-analysis showed that an increased risk of ischemic stroke occurred in the short-term after herpes zoster infection, whereas a significant relationship was not observed in the long-term after infection [13]. In addition, between 1% and 5% of patients with HIV develop stroke. Several indirect mechanisms (e.g., cardioembolism or opportunistic infection causing stroke) and direct mechanisms (e.g., HIV-associated vasculopathy) have been proposed [14]. While coronaviruses most frequently cause symptoms of the common cold, three coronaviruses can lead to much more severe respiratory infections, which have caused major outbreaks of fatal pneumonia in the 21st century: severe acute respiratory syndrome (SARS) in 2003, Middle East respiratory syndrome in 2012 and 2015, and coronavirus disease 2019 (COVID-19) this year. These coronaviruses may enter the brain through olfactory pathways and cause meningitis [15]. There have been cases of stroke in patients with SARS and COVID-19 [16-19], but an association of these coronaviruses with stroke is debatable, and possible mechanisms of stroke were varied including coagulopathy, vascular endothelial dysfunction, and large-vessel occlusion. One recent retrospective study showed that patients with more severe COVID-19 infection (according to respiratory status) had greater neurological involvement than those with milder infection; acute stroke was observed in five (5.7%) versus one (0.8%) patient among 213 cases of COVID-19 infection [17]. These coronaviruses have a high propensity to spread infection; thus, requiring a 14-day self-isolation quarantine program for contacts of infected patients, and there is currently no available treatment.
In conclusion, the causal relationship between infections and stroke remains unclear. In this study, we presented several infectious organisms for which causality is deemed relatively high. Cases presented in this study showed that the pathomechanisms of stroke differ between pathogens. Specifically, the distribution of cerebral infarction suggests that infections could be a direct cause of acute stroke. Examples discussed here include cerebral convexity probably due to vasculitis in bacterial meningitis, deep infarcts due to endarteritis and inflammation in neurocysticercosis, and exudates in tuberculous meningitis, as well as hemodynamic border zone infarct due to large vessel vasculitis by direct invasion of fungal infection. We propose that proper control of these organisms could prevent ischemic stroke.
Notes
AUTHOR CONTRIBUTIONS
Conception or design: OYB.
Acquisition, analysis, or interpretation of data: WL, JK, JZ, OYB.
Drafting the work or revising: WL, OYB.
Final approval of the manuscript: OYB.
Fig. 1.
A 62-year-old man with bacterial meningitis. Serial diffusion-weighted images (DWIs) show that initially infarcts were located at bilateral frontal convexity, but became extended after 5 days. The distribution of infarcts did not follow the vascular territory. Perfusion weighted image (PWI) shows hypoperfusion areas located in the cortical superficial regions. Leptomeningeal enhancement was most prominent over the infarcted cortex. Magnetic resonance angiogram (MRA) shows that large vessels were intact but distal branches were decreased. FLAIR, fluid attenuated inversion recovery.
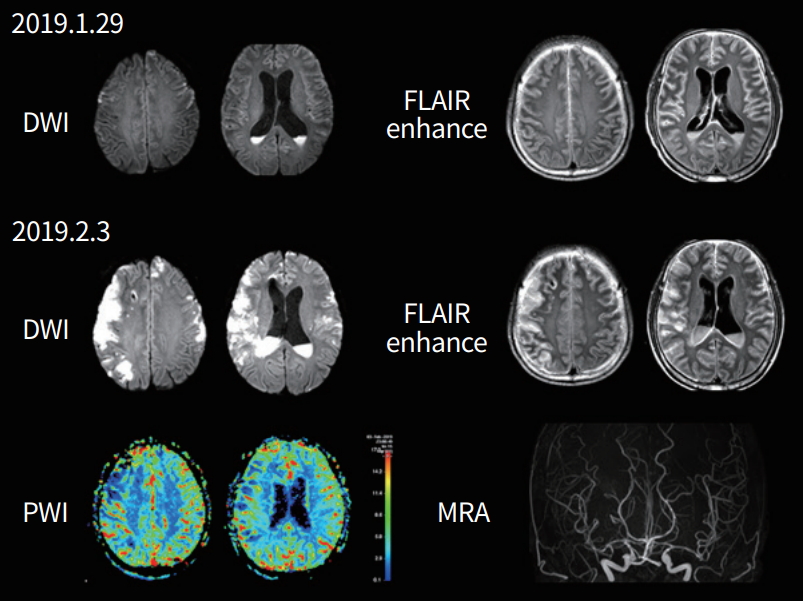
Fig. 2.
A 70-year-old woman with a fungal infection. Magnetic resonance image (MRI) at the time of complete ophthalmoplegia and ptosis, showing enhancing mass lesions in the left orbital area, but normal vessels on the magnetic resonance angiogram (MRA). Diffusion-weighted image (DWI) taken eight weeks later, showing acute infarcts in the left borderzone area and that the left distal internal carotid artery (arrow) was occluded on the MRA. Cranial MRI shows enhancing mass lesions on the left cavernous sinus, orbital apex, and optic nerves (arrowheads). Wall enhancement is observed in the left internal carotid artery (small arrow).
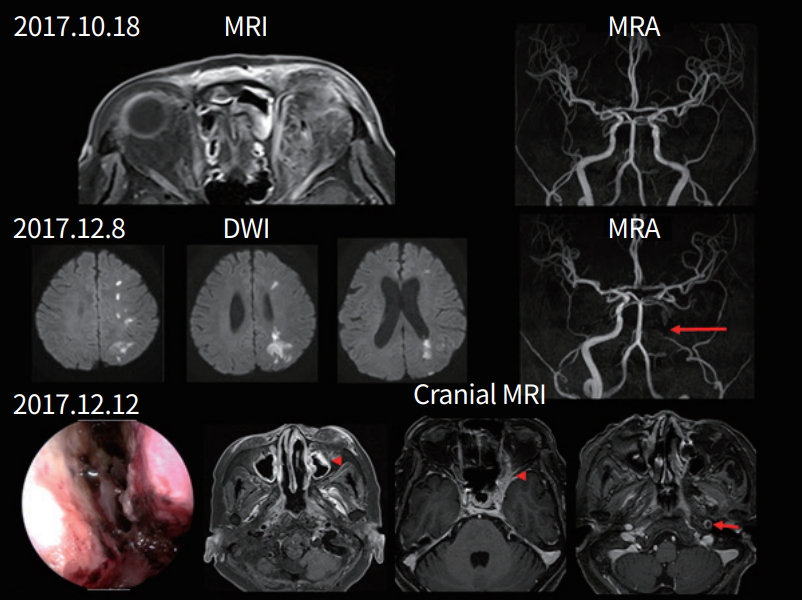
Fig. 3.
A 48-year-old woman with tuberculous meningitis. Diffusion-weighted image (DWI) at initial examination and follow-up showing acute infarcts on left medulla and right thalamus, respectively. Magnetic resonance angiography (MRA) showing normal large cervicocephalic vessels.
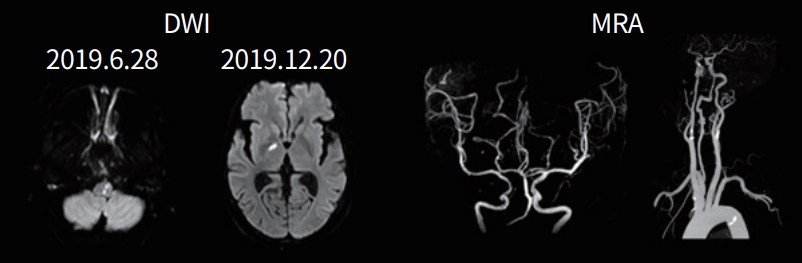
Fig. 4.
A 59-year-old man with neurocysticercosis. Diffusion-weighted image (DWI) shows acute infarcts on the right corona radiata. Magnetic resonance image (MRI) shows hydrocephalus and enhancing calcified cystic nodules in the cisternal space. Magnetic resonance angiogram (MRA) shows occlusion of the bilateral middle cerebral arteries and circular enhancement/thickening on the involved segments on high-resolution MRI (HRMRI) (arrowheads). Plain X-ray shows multiple calcifications in the leg muscles, suggesting cysticercosis.
REFERENCES
1. Fernandes BFS, Caramelli P. Ischemic stroke and infectious diseases in low-income and middle-income countries. Curr Opin Neurol 2019;32:43–8.


2. Jimenez M, Krall EA, Garcia RI, Vokonas PS, Dietrich T. Periodontitis and incidence of cerebrovascular disease in men. Ann Neurol 2009;66:505–12.



3. Zurru MC, Alonzo C, Brescacin L, Romano M, Camera LA, Waisman G, et al. Recent respiratory infection predicts atherothrombotic stroke: case-control study in a Buenos Aires healthcare system. Stroke 2009;40:1986–90.


4. Westendorp WF, Vermeij JD, Zock E, Hooijenga IJ, Kruyt ND, Bosboom HJ, et al. The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet 2015;385:1519–26.


5. Kalra L, Irshad S, Hodsoll J, Simpson M, Gulliford M, Smithard D, et al. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet 2015;386:1835–44.


6. Bang OY, Ovbiagele B, Kim JS. Nontraditional risk factors for ischemic stroke: an update. Stroke 2015;46:3571–8.


7. Fugate JE, Lyons JL, Thakur KT, Smith BR, Hedley-Whyte ET, Mateen FJ. Infectious causes of stroke. Lancet Infect Dis 2014;14:869–80.


8. Ionita CC, Siddiqui AH, Levy EI, Hopkins LN, Snyder KV, Gibbons KJ. Acute ischemic stroke and infections. J Stroke Cerebrovasc Dis 2011;20:1–9.


9. Schut ES, Lucas MJ, Brouwer MC, Vergouwen MD, van der Ende A, van de Beek D. Cerebral infarction in adults with bacterial meningitis. Neurocrit Care 2012;16:421–7.



10. Bang OY, Heo JH, Choi SA, Kim DI. Large cerebral infarction during praziquantel therapy in neurocysticercosis. Stroke 1997;28:211–3.


11. Wasay M, Khan M, Farooq S, Khowaja ZA, Bawa ZA, Mansoor Ali S, et al. Frequency and impact of cerebral infarctions in patients with tuberculous meningitis. Stroke 2018;49:2288–93.


12. Nair PP, Kalita J, Kumar S, Misra UK. MRI pattern of infarcts in basal ganglia region in patients with tuberculous meningitis. Neuroradiology 2009;51:221–5.



13. Lian Y, Zhu Y, Tang F, Yang B, Duan R. Herpes zoster and the risk of ischemic and hemorrhagic stroke: a systematic review and meta-analysis. PLoS One 2017;12:e0171182.



14. Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol 2012;11:878–90.



15. Dube M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol 2018;92:e00404–18.



16. Umapathi T, Kor AC, Venketasubramanian N, Lim CC, Pang BC, Yeo TT, et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS). J Neurol 2004;251:1227–31.




17. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:1–9.









