 |
 |
- Search
| Precis Future Med > Volume 6(2); 2022 > Article |
|
Abstract
Purpose
Despite the promising palliative effects of radiation treatment, few reports have studied the role of palliative tumor surgery (PTS) in patients with unresectable head and neck cancer (HNC). Thus, we aimed to present the outcomes of PTS in HNC, and suggest a possible surgical indication for PTS.
Methods
We retrospectively reviewed the medical records of 18 patients who underwent PTS for HNC between 2002 and 2017. PTS was defined as surgical debulking of tumor or surgery of loco-regionaltumors in patients with distant metastasis. As functional outcomes, we evaluated changes in pain, diet, respiration, and wound care before and after PTS.
Results
Squamous cell carcinoma was the common cancer type (72.2%), followed by salivary gland cancers and others. The median overall survival time was 17 months (95% confidence interval, 7.3 to 26.7). PTS significantly reduced the pain score (P= 0.013), and improved cancer-related wounds (P=0.003 in wound infection). Oral swallowing and respiration status did not change after PTS. The recurrent tumor at the operation bed was clinically detected at post-operative 1 to 2 months with intact skin (without wound problems). Of note, further chemotherapy or other additional cancer treatments was possible in 66.7% of patients with PTS (P=0.002).
Conclusion
PTS could provide a meaningful benefit to selected patients with incurable HNC, in terms of pain control and cancer wound management. Thus, PTS is a considerable option for selected HNC patients, based on the accurate evaluation of tumor extent along with multi-disciplinary consultation as well as patient counseling.
Advanced head and neck cancer (HNC) still causes significant cancer mortality from metastasis, even with recent advancements in cancer therapeutics [1,2]. To achieve better outcomes in advanced HNC, multi-modal treatments were developed. These treatments have been proven to be more effective and have become the standard of care [3,4]. Surgery is usually directed for resectable cases in advanced HNC, with the aim of achieving complete resection (R0) orinevitable micro-residual disease (R1), no gross residual disease (R2)[5].
Palliative surgery in cancer treatment involves various forms of interventions in incurable HNC [6-8]. To secure the airway, prophylactic tracheostomy is one of the most common procedures in patients with HNC, and endoscopic or fluorographic gastrostomy is another palliative method to improve patient’ nutrition and quality of life [8]. Palliative tumor surgery (PTS) specifically indicates the removal of cancer tissue to improve patient’ quality of life, but not complete eradication ofthe cancer[9,10].
Apart from palliative surgery, numerous articles have suggested the clinical usefulness of palliative radiation to control cancer-related pain in patients with HNC [11,12]. However, additional palliative radiation cannot always be performed for patients with HNC who have already received heavy irradiation, or for some tumors not responsive to radiation. Radiation can aggravate wound problems (infection, fistula, or bleeding from major vessels) in HNC patients with open cancer wounds.
In addition to improving patient comfort, a tailored palliative surgery can prolong patient survival in some cases [13]. Thus, it is important to refine the surgical indications of PTS for patients with HNC, under the multi-modal treatment strategies. Although radiation treatment has shown the promising palliative effects, few reports have addressed the role of PTS in patients with incurable HNC [11,12]. Thus, this retrospective case review was designed to present the outcomes of PTS in HNC, and to suggest surgical indications for which patients with HNC would most benefit.
We retrospectively reviewed the electronic medical records of patients who underwent PTS for HNC between 2002 and 2017. We defined PTS as (1) surgical debulking ofthe tumor in the primary site or neck, where complete tumor resection (R0) was not possible or(2) surgery to remove loco-regional tumors in a patient with distant metastasis (M1). Surgical procedures such as tracheostomy for airway maintenance or gastrostomy for nutritional support were excluded from the analysis.
During the 16-year study period, we identified 18 patients among 3,000 HNC surgeries, who met the inclusion criteria. The following clinical information was retrospectively obtained for each patient: age, gender, performance status, primary site,tumor pathology, initial and recurrent tumor-node-metastasis (TNM) stage (according to the American Joint Committee on Cancer [AJCC] 7th edition), treatments, and survival. Performance status was measured using the Eastern Cooperative Oncology Group (ECOG) scale [14]. The protocol of this retrospective study was approved by Institutional Review Board of Samsung Medical Center(IRB No. 2015-06-132), and the requirement for written informed consent was waived.
For functional outcomes, we focused on pre-surgery to post-surgery changes in pain, diet, respiration, and wound care. Post-operative data were collected at 1 to 2 months after PTS, when the surgical wounds had almost healed. Pain severity was measured using a numeric rating scale (NRS; 0, no pain; 10, maximum) [15]. Open wounds were re-evaluated by medical records and classified as persistent tumor bleeding, pus discharge orfoul odor with associated wound infection.
Swallowing function was categorized based on dietary status in the following groups: tube feeding, oral soft fluid diet, soft blended diet, and normal regular diet (NRD). Regarding airway maintenance, the status of airway in patients was divided into normal patent airway (without tracheostomy) or tracheostomy (including total laryngectomy). Overall survival after PTS was calculated from the time of PTS until the last follow-up or death from any cause.
Statistical analysis was performed using SPSS software version 20 (IBM SPSS, Armonk, NY, USA). Patient survival was calculated using the Kaplan-Meier method. Changes in pain scores were compared with a non-parametric paired test (before and after PTS) (McNemar test). The significance level for all statistical comparisons was set at P<0.05.
The patient characteristics are summarized in Table 1. Fifteen of the 18 (83%) subjects were male, and the median age was 59 years (range, 35 to 71). Regarding the primary site, the oral cavity ranked first (27.8%) followed by the larynx (22.2%) and unknown primary HNCs (16.7%). Squamous cell carcinoma was the most common histology (72.2%). Six patients (33.3%) had distant metastasis (M1) (five lung and one bone metastasis) and 16 patients (88.8%) had AJCC stage IV cancer at diagnosis. The 66.7% of patients (n=12) experienced recurrence after initial curative treatments (six surgery alone, five surgery with adjuvant radiation or chemoradiation, one definitive radiation). The remaining six patients (33.3%) were managed with initial palliative treatments (three surgery with chemotherapy, two chemoradiation, one surgery with radiation). All patients had stage IV cancer and 12 patients (66.7%) had distant metastasis at the time of PTS.
At the time of PTS, ten patients had metastasis in lung, one in bone, and the other one in the lung and skull base. PTS was performed for the following purposed: (1) debulking of large tumors (55.6%); (2) wound management(22.2%); (3) pain control (11.1%); and (4) improvement of oral swallowing (11.1%) (Table 1).
Three patients were excluded from the survival calculation due to short follow-up duration. Among the 15 patients, eight patients died due to cancer-related problems (53.3%). There were four deaths of cancer-unrelated reasons (heart, respiratory, hepatic failures, and accident), and three were lost to follow-up or censored at the time of data collection. The median overall survival time was 17 months (95% confidence interval, 7.3 to 26.7)in 15 patients who received PTS (Fig. 1).
Although PTS was primarily performed to manage cancer-related pain in only two patients, 17 patients (94.4%) experienced a decrease in pain score after PTS. The mean preoperative NRS score was 3.21 (range, 0 to 8), but it was reduced to 1.14 (range, 0 to 4) after the operation, representing a significant decrease (P=0.013)(Fig. 2).
Changes of the clinical variables after PTS are summarized in Fig. 3. Before surgery, 16 patients (88.8%) had received supportive care alone (duration, 1.0 to 7.5 months) without any cancer treatments. However, 10 patients were able to receive chemotherapy or other additional treatments after PTS.
Approximately 40% of the patients suffered from bleeding, discharge, and foul odor due to loco-regional tumor progression. The wound problems were well managed with PTS, suggesting that PTS improved quality of life of these patients.
In addition, we investigated the post-operative changes in diet(swallowing) and respiration. Eight patients (44.4%) were able to take NRD before and after surgery. Fifteen patients (83.3%) had a normal oro-laryngo-tracheal airway before surgery and one additional transient tracheostomy was required with PTS. Overall swallowing and respiratory functions were similar between pre-operative and post-operative status. Rather, those functions were transiently decreased at the immediate post-operative period and then slowly returned to the pre-operative status.
Advanced stage HNC at presentation or metastatic/recurrent HNC still signifies a poor patient prognosis, even with recent advances in diagnostic modalities and multi-modal treatments [16]. A longitudinal follow-up study of untreated patients with HNC (91% of whom were in stage IV) showed a median survival of approximately 100 days [17]. Previously, we also reported survival outcomes of HNC patients with distant metastasis, and we found that the median survival of HNC patients with multiple distant metastases of high glucose uptake on positron emission tomography (standard uptake value >6.3) was only 10 months [18]. The symptoms of these patients worsened gradually over a period of a few months, and the patients suffered from physical and psychological distress [9]; thus, many patients needed palliative treatment. Several studies have suggested the clinical usefulness of palliative radiation or chemotherapy in patients with HNC [19-21]; however, few reports have studied the role of PTS in patients with incurable/unresectable HNC [22]. Therefore, we aimed to present the outcomes of palliative surgery for incurable HNC, and to suggest possible surgical indications for PTS.
Palliative treatments in patients with HNC, including gastrostomy and tracheostomy, are designed primarily to relieve pain, support nutrition and secure the airway. Apart from gastrostomy and tracheostomy, PTS in HNC seems to play a minor role in patient management compared with supportive care, chemotherapy, or radiation [23]. Palliative chemotherapy response rate typically range from 20% to 50%; longer survival rates can be achieved depending on the drug regimen [19,21]. There have been conflicting reports about the effects of palliative chemotherapy for HNC on quality of life [20,24]. However, no well-designed randomized clinicaltrial has been undertaken to definitively show a survival benefit of palliative chemotherapy over the best supportive care for these patients [25]. In reality, it appears to be difficult to manage local cancer-related wound problems during chemotherapy.
The primary goal of radiotherapy for patients with incurable HNC is effective palliation, i.e., improved quality of life, symptom relief, and fewer complications, all with minimal toxicity [26]. Numerous studies have suggested that palliative radiotherapy could achieve high rates of symptom relief in HNC patients, even with only marginal or no survival benefit [27-29]. However, loco-regionally advanced HNC represents a significant treatment challenge due to the close proximity of tumors to critical normal tissues, such as the skull base, spinal cord, salivary glands, cranial nerves, major blood vessels and the organs of speech, swallowing,respiration, and hearing [28]. Likewise, significant overlap can exist between the presenting symptoms and radiation-related toxicity, making decisions about palliative radiotherapy challenging. Moreover, high quality evidence is lacking for determining the optimal palliative radiation regimen (time,dose, and fractionation).
It is worth noting that patients with recurrent/metastatic HNC can receive maximal acceptable doses of radiation for surrounding tissue and organs over multiple courses of radiotherapy. Re-irradiation could carry significant potential risks and complications [25].
In our study, eight out of the 18 patients received primary radiation treatment for their disease and two of whom underwent additional irradiation after relapsing. Eventually, three patients suffered from complications due to radiotherapy (radio-necrosis in two and oro-cutaneous fistula in one). It appears to be very risky to apply radiation to wounds contaminated with cancer cells through the skin or mucosa.
Therefore, PTS could be a very good alternative treatment when patients with incurable HNC have previously had full-dose radiotherapy, the tumor seems to be unresponsive to radiotherapy, or the patient had cancer-related wound problems. One desired outcome of PTS is to reduce the size of the mass, since large masses, such as unresectable laryngopharyngeal tumors, cause obstructive speech, swallowing, and breathing. Tumor debulking and reconstruction could be also valuable for exophytic neck/cutaneous masses, with minimal surgical morbidity [8]. Although the survival rate might not improve, surgical management of foul odor, pain, bleeding, and infection associated with skin wound problems has the potentialto improve patient quality of life [30]. One study presented the outcomes of surgical interventions for symptoms arising from advanced HNC, and it included debridement and debulking, mandibulectomy, maxillectomy, the use of free flap reconstruction and major vessel ligation [9]. The authors suggested that surgical palliation can be performed safely while achieving symptom relief in a highly selected group of patients. Similarly, half of the patients in our series experienced complete pain relief after PTS and most of the wound problems were resolved, leading to improved quality of life in these patients.
Another question in the PTS is how long PTS is effective in the management of symptom in patients with incurable HNC. It is hard to draw any generalized conclusion from our series; however, the remaining tumor seems to re-grow at post-operative 1 to 2 months with an intact skin (without wound problems) (Fig. 4). Thus, PTS should be followed by an adequate reconstruction procedure notto delay surgical wound healing over 1 month, and the additional cancer treatment within 2 months after PTS appears to be appropriate.
Some limitations of this study include its retrospective descriptive nature and the lack of evaluation tools to assess symptoms and response to PTS. Moreover,the number of patients included in this study was too small to draw definite conclusions. In addition, differences among the patients regarding symptoms, extent of tumors and performance status made it difficult to set common surgical indications for PTS. Therefore, it is essential to determine an individualized PTS plan through accurate evaluation, multidisciplinary cooperation, and patient consultation. Of particular note, successive treatments after PTS such as radiotherapy, chemotherapy or targeted molecular therapy could be applied in patients who had undergone supportive care only. This finding suggests the possibility of improving patient survival. However, large prospective comparative studies are needed to validate our preliminary results.
In conclusion, PTS could provide a meaningful benefit to selected patients with incurable HNC, in terms of pain control and cancer wound management. In addition, PTS could provide an opportunity for the subsequent treatment for some patients who were initially not amenable to the further treatment. Thus, PTS is a considerable option for selected HNC patients, based on the accurate evaluation of tumor extent, multi-disciplinary consultation, and patient counseling.
Notes
AUTHOR CONTRIBUTIONS
Conception or design: YSC, HSJ.
Acquisition, analysis, orinterpretation of data: EL, HJ,DO.
Drafting the work orrevising: YSC,DO.
Final approval ofthe manuscript: YSC, EL, HJ,DO, HSJ.
ACKNOWLEDGEMENTS
This work was supportedby SamsungBiomedicalResearch Institute grant (SMX1170401), and the National Research Foundation of Korea grant funded by Korea MEST (2015R1D1A1A09 056771).
Fig. 1.
Overall survival of patients with head and neck cancer after palliative tumor surgery. CI, confidence interval.

Fig. 2.
Palliative tumor surgery significantly reduced subjective pain scores in enrolled patients. NRS, numeric rating score of pain severity (0= no pain to 10= worst pain imaginable).

Fig. 3.
Changes of clinical variables after palliative tumor surgery in patients with incurable head and neck cancer. The left and right bar graphs in each box indicate the pre-surgery and post-surgery status, respectively. (A) Further cancer treatment after palliative tumor surgery for incurable head and neck cancer. (B) Control of wound problems by palliative tumor surgery. Changes in oral diet (C) and respiratory airway (D) after palliative tumor surgery.
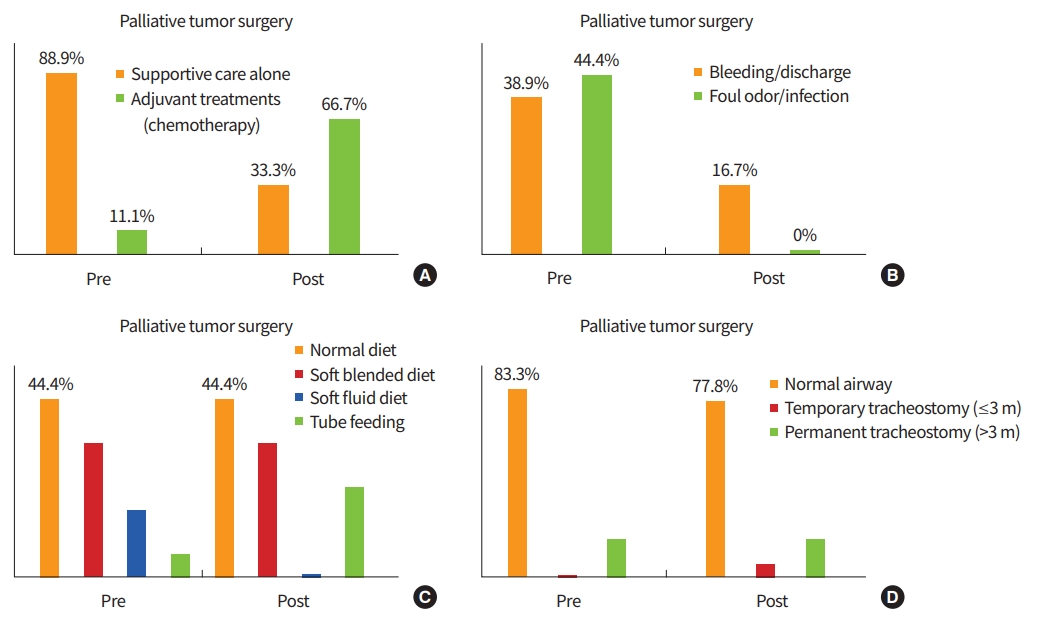
Fig. 4.
Representative cases of palliative tumor surgery. (A) A patient presented with large recurrent/metastatic tumors in the right postauricular area and suffered from skin ulceration, foul odor and tumor bleeding. Palliative tumor surgery was performed to remove skin penetrating large tumors and the wound was reconstructed with a free flap; however, tumors were left around right internal carotid artery and right vertebral artery. At post-operative 2 months, it was detected that the size of the remaining tumors increased, and new metastatic nodules were found in the axillary lymph nodes and sternum. The patient still had no cancer-related wound problems and the overlying skin (flap) was intact. This allowed the patient to receive an additional chemotherapy. (B) The tumor arising from the frontal sinus and forehead invaded the frontal lobe with dura and the left eye. Radiation did not work well, and the size of tumor increased even during radiation treatment. Palliative tumor surgery resected most of tumors, but tumor cells were left on the frontal lobe dura and the right eye periorbita; then, a free flap covered the surgical defect. At post-operative 2 months, re-growth of tumors in the right eye and metastatic nodules were detected, without a cancer-related wound problem (arrow: recurrent or metastatic tumor).

Table 1.
Clinical characteristics of the subjects enrolled in this study (n=18)
REFERENCES
2. Cooper JS, Porter K, Mallin K, Hoffman HT, Weber RS, Ang KK, et al. National Cancer Database report on cancer ofthe head and neck: 10-year update. Head Neck 2009;31:748–58.


3. Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist 2010;15:994–1001.



4. Sacco AG, Cohen EE. Current treatment options for recurrent or metastatic head and neck squamous cell carcinoma. J Clin Oncol 2015;33:3305–13.


5. Choong N, Vokes E. Expanding role ofthe medical oncologistin the management of head and neck cancer. CA Cancer J Clin 2008;58:32–53.


6. Chen H, Roberts JR, Ball DW, Eisele DW, Baylin SB, Udelsman R, et al. Effective long-term palliation of symptomatic, incurable metastatic medullary thyroid cancer by operative resection. Ann Surg 1998;227:887–95.



7. Tabaee A, Nyquist G, Anand VK, Singh A, Kacker A, Schwartz TH. Palliative endoscopic surgery in advanced sinonasal andanterior skull base neoplasms. Otolaryngol Head Neck Surg 2010;142:126–8.


8. Roland NJ, Bradley PJ. The role of surgery in the palliation ofheadandneck cancer. Curr Opin Otolaryngol Head Neck Surg 2014;22:101–8.


9. Chan JY, To VS, Wong ST, Wei WI. Quality of dying in head and neck cancer patients: the role of surgical palliation. Eur Arch Otorhinolaryngol 2013;270:681–8.


10. McCahill LE, Krouse R, et al. Indications and use of palliative surgery-results of Society of Surgical Oncology survey. Ann Surg Oncol 2002;9:104–12.


11. Wu JS, Wong R, Johnston M, Bezjak A, Whelan T, Cancer Care Ontario Practice Guidelines Initiative Supportive Care Group. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat OncolBiol Phys 2003;55:594–605.

12. Vargo JA, Ferris RL. Stereotactic body radiotherapy as primary treatment for elderly patients with medically inoperable head and neck cancer. Front Oncol 2014;4:214.



13. Ledeboer QC, van der Schroeff MP, Pruyn JF, de Boer MF, Baatenburg de Jong RJ, van der Velden LA. Survival of patients with palliative head and neck cancer. Head Neck 2011;33:1021–6.


14. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET. . Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55.


15. Turk DC, Rudy TE, Sorkin BA. Neglected topics in chronic pain treatment outcome studies:determination of success. Pain 1993;53:3–16.


16. Huang SH, Perez-Ordonez B, Weinreb I, Hope A, Massey C, Waldron JN, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol 2013;49:79–85.


17. Kowalski LP, Carvalho AL. Natural history of untreated head and neck cancer. Eur J Cancer 2000;36:1032–7.


18. Cho JK, Hyun SH, Choi JY, Choi N, Kim MJ, Lee SH, et al. Prognostic significance of clinical and 18 F-FDG PET/CT parameters for post-distant metastasis survival in head and neck squamous cell carcinoma patients. J Surg Oncol 2016;114:888–94.



19. Guntinas-Lichius O, Ruhlow S, Veelken F, Klussmann JP. Quality of life during first-line palliative chemotherapy for recurrent and metastatic head and neck cancer with weekly cisplatin and docetaxel. JCancerResClin Oncol 2009;135:901–8.

20. Stewart JS, Cohen EE, Licitra L, Van Herpen CM, Khorprasert C, Soulieres D, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol 2009;27:1864–71.

21. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–27.


22. Watson JC, Ridge JA. Surgical management of local and regional recurrent head and neck squamous cell carcinoma. Curr Opin Oncol 1998;10:207–12.


23. Stirland DL. Analytical methods for studying intratumoral drug delivery in solid tumors [dissertation] Salt Lake City (UT): The University of Utah; 2016.
24. Rivera F, Garcia-Castano A, Vega N, Vega-Villegas ME, Gutierrez-Sanz L. Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther 2009;9:1421–8.


25. Mehanna H, Kong A, Ahmed SK. Recurrent head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol 2016;130(S2):S181–90.



26. Stevens CM, Huang SH, Fung S, Bayley AJ, Cho JB, Cummings BJ, et al. Retrospective study of palliative radiotherapy in newly diagnosed head and neck carcinoma. Int J Radiat OncolBiol Phys 2011;81:958–63.

27. Chen AM, Vaughan A, Narayan S, Vijayakumar S. Palliative radiation therapy for head and neck cancer: toward an optimal fractionation scheme. Head Neck 2008;30:1586–91.


28. Agarwal JP, Nemade B, Murthy V, Ghosh-Laskar S, Budrukkar A, Gupta T, et al. Hypofractionated, palliative radiotherapy for advanced head and neck cancer. Radiother Oncol 2008;89:51–6.









