 |
 |
- Search
| Precis Future Med > Volume 5(4); 2021 > Article |
|
Abstract
The development of treatment options has revolutionized the prognosis of inflammatory bowel disease (IBD). However, a particular group of patients still experience therapeutic failure or drug side effects. Although the high inter-patient variability in therapy is associated with clinical factors, including age, disease behavior, and disease duration, they attribute only a small proportion of inter-individual variability. Thus, pharmacogenetics evaluating associations between specific genetic variations and drug responses or side effects have focused on optimizing therapeutic efficacy and minimizing toxicity in IBD treatment. Thiopurine S-methyltransferase (TPMT) and nudix hydrolase 15 (NUDT15) are well-established predictive markers of thiopurine-induced myelosuppression. Low TPMT activity is related to increased 6-thioguanine nucleotide levels, subsequently leading to myelotoxicity. NUDT15 variants are strongly associated with thiopurine-induced early leukopenia in Asians, with a lower incidence of TPMT-deficient allele. The Korean Association for the Study of Intestinal Diseases guidelines recommend pretreatment determination of NUDT15 genotypes, especially in East Asians, and NUDT15 R139C measurement has been approved for clinical use since 2019. Several studies have attempted to identify powerful genetic markers for personalized medicine. In this article, we review the identified pharmacogenetics of currently available drugs, focusing on 5-aminosalicylic acid, glucocorticosteroids, thiopurines, and anti-tumor necrosis factor-alpha agents.
Inflammatory bowel diseases (IBDs), primarily Crohn’s disease (CD) and ulcerative colitis (UC), are multifactorial diseases that develop aberrant immune responses in the intestines involving environmental factors, genetic variants, and intestinal dysbiosis [1-3]. Considering the disease characteristics of chronic relapsing inflammation with progressive complications, lifelong treatment strategies to prevent complications and maintain long-term remission are required. The development of treatment options has revolutionized the overall prognosis of IBD. Conventionally, 5-aminosalicylic acids (5-ASAs) are prescribed as first-line agents in patients with mild to moderate disease; glucocorticosteroids are prescribed for remission induction in moderate to severe disease and thiopurines for maintaining remission [4,5]. In addition, anti-tumor necrosis factor-α (anti-TNFα) agents have been shown to be effective for both inducing remission and maintenance in conventional therapy refractory disease [4], and anti-TNFα therapy has replaced treatment strategy from symptom control to mucosal healing [6-8]. However, a particular group of patients still experience therapeutic failure or adverse events. The high inter-patient variability in therapy is associated with clinical factors, including age, disease behavior, and disease duration [9,10]. However, they attribute only a small proportion of interindividual variability, and currently, genetic variation has been focused on the implementation of personalized IBD treatment. Pharmacogenetics evaluates the associations between specific genetic variations and drug responses to optimize therapeutic efficacy and minimize toxicity [9]. Recently, extreme phenotype selection strategy has been adopted as a methodology for pharmacogenetic analysis [11,12]. This strategy has identified relevant biological factors with effectiveness by reducing the sample size required for molecular studies, and interpreting the large amount of data generated by high-throughput techniques [13,14]. In this article, we review the identified pharmacogenetics of currently prescribed drugs, focusing on 5-ASAs, glucocorticosteroids, thiopurines, and anti-TNFα.
5-ASA is the first-line treatment for the induction and maintenance of IBD remission [4,15,16]. 5-ASA acts locally on the colonic mucosa and reduces inflammation by activating nuclear receptors involved in the control of inflammation, γ-form peroxisome proliferator-activated receptors (PPAR-γ) which inhibit several target genes, including nuclear factor kappa B (NFκB), leukotrienes, prostaglandins, and interleukin (IL)-1 [17,18]. Thus, mucosal concentrations of 5-ASA are important to intensify drug effects [15]. In the intestinal mucosa, 5-ASA is poorly absorbed into the systemic circulation and rapidly N-acetylated mainly by the enzyme N-acetyl-transferase 1 (NAT1), and to a small degree by NAT2 [19]. Genetic polymorphisms in these enzymes lead to rapid or slow acetylation, and such variants are found in over 50% of Caucasians as slow acetylators [20].
However, NAT genotypes are unlikely to play a role in predicting 5-ASA responses. In 78 UC patients, the genetic variations of NAT1 (NAT1*3, *4, *10, and *11 allele) were not associated with response or side effects to mesalamine [21]. In addition, in a population-based cohort of patients with UC, NAT1, and NAT2 genotypes did not predict clinical response or toxicity to 5-ASA [22]. Recently, a study evaluated the association between 5-ASA concentration, 5-ASA formulation, NAT genotype, and microbiome in quiescent UC patients receiving 5-ASA monotherapy. Mezavant (Shire Pharmaceutical Contracts Ltd., in partnership with Cosmo SpA, Milan, Italy) provided higher 5-ASA mucosal concentration than Pentasa (Ferring Pharmaceuticals, Saint-Prex, Switzerland) (2.39 ng/mg vs. 0.57 ng/mg, P= 0.033). Although patients with NAT1 slow acetylators had higher 5-ASA serum concentrations, no influence was found in terms of mucosal 5-ASA concentration. Interestingly, mucosal 5-ASA concentration was positively related with gut microbial diversity and composition [23].
PPAR-γ gene expression, a key mediator of inflammation, was decreased in the active UC group compared with that in the UC in remission (P= 0.001) and control groups (P= 0.001) [24]. Increased expression of the PPAR-γ gene was associated with a milder clinical course (odds ratio [OR], 0.05; P≤ 0.001) [24] and milder endoscopic disease activity in active UC [25].
Considering the need for long-term maintenance of 5-ASA treatment, a genome-wide association study was performed to identify the risk factors associated with rare idiosyncratic nephrotoxicity in IBD patients. In this study, the median time for renal injury was 3 years after 5-ASA administration. The strongest association signal was in the human leukocyte antigen (HLA) region (rs3135349, OR, 2.04; P= 1× 10−7), and the top single nucleotide polymorphism (SNP) after dedicated HLA imputation was rs3135356 (OR, 2.0; P= 1× 10−7). When limited to biopsy-proven interstitial nephritis cases, the most associated HLA allele was HLA-DRB1*03:01 (OR, 2.76; P= 5× 10−7) [26]. However, its clinical utility is not recommended because of its high frequency of risk alleles and low frequency of nephrotoxicity [9].
Recently, a novel genetic factor associated with mesalamine allergy has been reported. Mesalamine allergy, which is characterized by high fever, worsening diarrhea, or bloody stool makes it difficult to distinguish from exacerbation of IBD [27]. These symptoms are more common in UC than those in CD, with varying incidence from 2.1% to 24% [16,27,28]. Mesalamine allergy is considered a type IV allergic reaction mediated by antigens and T cell-recognizing antigens (especially T helper 1 cells) [27]. Although the drug-induced lymphocyte stimulation test has been proposed as a diagnostic test, the positive rate of mesalamine intolerance was 24%, suggesting the presence of multiple mechanisms besides allergic reaction [28]. In an effort to identify the genetic background of mesalamine intolerance, the Japanese study team identified rs144384547 (upstream of regulator of G-protein signaling 17 [RGS17]) to be significantly associated with mesalamine-induced fever and diarrhea (OR, 11.2; P= 7.21e-09) with an estimated heritability of 25.4% [29]. Although information regarding the role of RGS17 in IBD or mesalamine pharmacogenetics is unavailable, RGS17 has been reported as a negative modulator of G-protein-coupled receptor signals in several types of cancer [30]. The combined genetic and clinical prediction models which could overcome the limitation of single genetic polymorphism analysis, yielded a higher area under the curve than the polygenic risk score or clinical model alone (area under the curve, 0.89; sensitivity, 71.4%; specificity, 90.8%) [29]. Pharmacogenetic candidates for the management of inflammatory bowel disease are summarized in Table 1.
Systemic glucocorticoids are the first-line conventional drugs for inducing remission of moderate to severe exacerbation of IBD [4]. Potent and rapid anti-inflammatory effects follow binding of the intracellular glucocorticoid receptor and inhibition of T cell activation and cytokine secretion [31]. However, approximately 20% of patients are resistant to glucocorticoids, and 30% to 40% of patients acquire dependency [32]. In a population-based study of patients with IBD, immediate outcomes for CD and UC were as follows: complete remission, 58% and 54%; partial remission, 26% and 30%; and no response, 16% and 16%. One year after the first course of treatment with corticosteroids, 32% of CD patients and 49% of UC patients had prolonged responses, whereas 28% of CD patients and 22% of UC patients showed corticosteroid dependence [33].
Glucocorticoids passively diffuse across the plasma membrane and activate a cytosolic glucocorticoid receptor which leads to repression or induction of the expression of several inflammatory genes [34]. As the mechanism of action of glucocorticoids is complex, glucocorticoid resistance might occur at several distinct molecular levels [35].
The glucocorticoid receptor variant is the most well-researched candidate involved in glucocorticoid pharmacogenetics. Polymorphisms of the glucocorticoid receptor gene nuclear receptor subfamily 3, group C, member 1 (NR3C1) impair the formation of the glucocorticoid-glucocorticoid receptor complex and alters transactivation or transrepression processes. In patients with steroid-resistant UC, the expression level of glucocorticoid mRNA in the intestinal mucosa is decreased [36]. To date, three polymorphisms, TthIII1, ER22,/,23EK, and GR-9β, are known to be associated with reduced sensitivity to endogenous or exogenous glucocorticoids. However, the role of these polymorphisms in IBD treatment is not well established. In 119 pediatric patients with IBD, no association was observed between ER22/23EK polymorphisms and glucocorticoid response [37].
Among SNPs of the NR3C1 gene, the Bcl1 and N363S polymorphisms have been reported to have increased glucocorticoid sensitivity [34]. Bcl1 polymorphism consists of a C> G substitution, 646 nucleotides downstream from exon 2 [10,38], and heterozygous and homozygous carriers of the G allele showed hypersensitivity to glucocorticoids [38]. In a pediatric IBD study, a higher frequency of Bcl1 mutation was associated with glucocorticoid hypersensitivity in the glucocorticoid response group than that in the dependent group (OR, 3.61; 95% confidence interval [CI], 1.44 to 9.01; P0.006/0.030) [37]. Although the N363S polymorphism was found to have an influence on increased sensitivity to glucocorticoids in vivo [39], so far, there has been no association with clinical response to glucocorticoids in IBD patients [37]. However, a meta-analysis reported that glucocorticoid receptor gene polymorphisms, including Bcl1, ER22/23EK, and N363S were not associated with glucocorticoid resistance in IBD [40], and further well-designed cohort studies are needed.
Variants in the principal effectors of glucocorticoids are also considered to play a role in interindividual differences in clinical drug response. The most well-identified candidate is the drug efflux pump P-glycoprotein (Pgp) encoded by the multidrug resistance gene 1 (MDR1 gene)/ATP binding cassette subfamily B member 1 (ABCB1) gene [34,41]. Pgp, expressed on the apical surface of lymphocytes and intestinal epithelial cells [1], actively extrudes structurally unrelated substances from cells, resulting in reduced intracellular concentrations of glucocorticoids [10]. Among more than 50 polymorphisms described in the MDR1 gene, a non-coding SNP in exon 26 (C3435T) and tri-allelic G2667T/A have been considered as factors for IBD susceptibility [42-44] and response to glucocorticoids [42,45,46]. However, the results of these studies are conflicting, and further international efforts or meta-analyses are required.
Pro-inflammatory cytokines involved in the pathogenesis of IBD have been suggested to interfere with glucocorticoid receptor signaling [47]. The 308G >A (rs1800629) polymorphism of the TNFα gene is associated with steroid dependency in CD [48]. IL-1β polymorphism involved in an inflammatory cascade had no correlation with steroid response, whereas polymorphism of caspase-1 showed a low response to corticosteroids [49].
A recent study analyzed 21 genes associated with glucocorticoid receptors, transporters, cytokine genes, chaperones/co-chaperones, and kinases using the amplicon next-generation sequencing method and reported four genes as pharmacogenetic biomarkers to determine variable reactions of glucocorticoids. In this study, the c.1088A> G polymorphism in the NR3C1 gene was associated with glucocorticoid resistance (P= 0.002) and variant c.241+ 6A> G of the FK506 binding protein 5 (FKBP5) gene with glucocorticoid sensitivity (P = 0.040). In CD, the change c.2685 +49T >C of the ABCB1 gene was linked to glucocorticoid resistance (P=0.034), whereas the deletion c.306-7delT in the MAPK14 gene was linked to an adverse therapeutic effect (dependency and resistance, P=0.041) in UC [31].
Thiopurines have been the mainstay treatment for IBD for the maintenance of long-term remission and steroid-sparing agents [50-52]. They also reduce the risk of anti-drug antibodies and increase the efficacy of anti-TNFα agents [53]. Oral administration and relatively lower costs are their advantages in clinical usage [54]. However, variability in efficacy and toxicity related to inter-individual pharmacokinetic differences and genetic polymorphisms can result in drug discontinuation. Approximately 25% of patients have to stop thiopurine due to adverse reactions including myelosuppression, hepatotoxicity, gastric intolerance, and increased risk of malignancy [55].
Myelosuppression is the most common and serious dosedependent adverse effect of thiopurines, which further cause infection and mortality [54,56]. Leukopenia, the most common form of thiopurine-induced myelosuppression, develops at any time during treatment, ranging from 12 days [57] to 27 years [58]. Severe cases usually occur within the first month [56]. The incidence of leukopenia is higher in East Asian IBD populations including Koreans (31.2% to 39.6%) [59,60], Chinese (15.6%) [61], and Japanese (20.8%) [62] than that in Caucasians (5%) [63,64], which reflects the different genetic contributions of thiopurine metabolism.
Thiopurine drugs, azathioprine and its analog, 6-mercaptopurine, are prodrugs that require multiple enzymatic processes to become active metabolites [65]. The synthetic pathway of the active metabolites is in competition with inactivation pathways mediated by xanthine oxidase or thiopurine S-methyltransferase (TPMT). Thus, patients with the TPMT mutation have an increased risk of thiopurine-induced leukopenia [66], and the U.S. Food and Drug Administration recommends pretreatment TPMT test [51]. Low TPMT activity is related to increased 6-thioguanine nucleotide levels by increasing inosine monophosphate dehydrogenase activity, which subsequently leads to myelotoxicity. The major alleles accounting for TPMT deficiency were TPMT*2, TPMT*3A, and TPMT*3C [67]. In Caucasians, 0.3% are homozygous for low enzyme activity (complete deficiency), 11% are heterozygous (partial deficiency), and 90% are homozygous for high enzyme activity (high activity) [68]. However, Asians have a lower incidence of TPMT-deficient alleles, with less than 5% of the population having at least one defective allele [60,69,70]. In addition, TPMT polymorphisms are related to only 10% to 25% of the overall thiopurine toxicity [71,72], which limits the value of pretreatment TPMT genotype assessment in Asians [73].
In an effort to find more predictable genetic markers, Yang et al. [69] reported that a missense variant in the nudix hydrolase 15 (NUDT15) (p.Arg139Cys) gene is strongly associated with thiopurine-induced early leukopenia in patients with CD (OR, 35.6; Pcombined=4.88 ×10-94). NUDT15 dephosphorylates the thiopurine active metabolites, thioguanosine triphosphates (TGTP) and deoxy-thioguanosine triphosphate (TdGTP), and prevents the binding of TGTP to Rac1 and the incorporation of deoxy-TGTP into DNA [74]. Thus, the deficiency of NUDT15 increases the levels of thiopurine active metabolites and increases drug toxicity [74]. Compared with the noncarriers of risk alleles, patients with homozygote variants had a lower tolerated dose (0.86 mg/kg/day vs. 1.53 mg/kg/day, P =4.93 ×10-11), shorter interval from onset of therapy to leukopenia (19 days vs. 135 days, P=1.03×10-17), and more severe grade (grade 3 or 4) leukopenia (14 patients vs. 4 patients, P=4.85×10-19) [69].
Compared to TPMT, NUDT15 variants are most frequent in East Asians (9.8%), followed by Hispanics (3.9%), and rarely in Europeans (0.2%) [75]. Thus, the impact of pretreatment NUDT15 tests in preventing early leukopenia is significant, particularly in Asians [54]. The Korean Association for the Study of Intestinal Diseases guidelines recommends pretreatment determination of NUDT15 genotypes, especially in East Asians [52]. In addition, NUDT15 R139C measurement has been approved for clinical use in Japan since 2019 [76].
Recently, a novel coding variant, fat mass and obesity-associated (FTO) p.A134T (rs79206939), using a genome-wide association study was found in IBD patients [77]. FTO belongs to the AlkB family of Fe(II)/α-ketoglutarate-dependent dioxygenases and demethylates a nucleotide [54]. The demethylation function of FTO engages in the abrogation of methyl-TIMP, a thiopurine metabolite, which is a potent purine synthesis inhibitor [78], and the repair of impaired DNA or RNA [79]. Leukopenia occurred in 66.7% of patients with FTO p.A134T and in 32.4% of patients with wild-type FTO. The nucleotide demethylase assay confirmed that the p.A134T variation reduced FTO activity by 65% [77]. Based on this study, a randomized controlled study was conducted to evaluate the value of a tailored therapy with pretreatment genotyping for three genes (TPMT, NUDT15, and FTO) to prevent thiopurine-induced myelosuppression in IBD patients [80]. Pretreatment genotyping of one NUDT15 variant (rs116855232), one FTO variant (rs79206939), and three common TPMT variants (rs1800460, rs1800462, rs1142345) effectively reduced thiopurine-related myelosuppression compared to that in the non-genotyping group (15.4% vs. 42.1%, P= 0.001). Pretreatment genotyping also reduced the number of thiopurine discontinuations or dose reductions during thiopurine administration. Considering the higher frequency of FTO p. A134T in Koreans (5.1%) compared with that in the Western populations (< 0.1%) [77], FTO could be a candidate to predict the risk of thiopurine-induced myelosuppression in East Asian patients [54]. Therefore, further research is necessary.
Besides myelosuppression, pancreatitis is another major thiopurine-induced adverse event affecting 2% to 7% of patients [81,82]. Thiopurine-induced pancreatitis is dose-independent and unpredictable and almost always leads to drug withdrawal [82]. In 2014, a genome-wide association study identified a 2.59-fold increased risk of pancreatitis in IBD patients who had the class II HLA gene region polymorphism (rs2647087 mapped to the HLA-DQA1*02:01-HLA-DRB1*07:01) [83], and which was confirmed in another cohort. The risk of pancreatitis was 0.53% for wild-type (A/A), 4.25% (OR, 4.19; 95% CI, 1.02 to 36.45; P= 0.044) for heterozygous (A/C), and 14.63% (OR, 15.83; 95% CI, 3.80 to 145.26; P= 0.0001) for homozygous variant (C/C) patients [81].
Based on previous studies, the following protocol was proposed for the dosing of thiopurines in IBD patients (Fig. 1). Thiopurine therapy should be personalized based on the individual pharmacogenetic results.
Anti-TNFα agents, mainly infliximab (IFX) and adalimumab, are the most effective treatments for inducing and sustaining clinical and endoscopic remissions in both UC and CD patients [84-88]. Anti-TNFα agents rapidly improve inflammation with onset of effects within 2 weeks [10] and have advanced clinical outcomes including reduction in hospitalizations and surgeries, steroid-free remission, better quality of life, and healing of fistulizing CD [1,89]. Despite their high efficacy, one-third of patients were unresponsive (primary nonresponse), and an additional one-third who initially responded to therapy experienced relapse (loss of response) [90-92]. The formation of anti-drug antibodies, that is, immunogenicity, is an important determinant of therapeutic failure [93,94]. Approximately 65% of patients treated with IFX and 38% of patients treated with adalimumab develop have anti-drug antibodies despite concomitant use of immunosuppressive agents such as thiopurines that reduce immunogenicity [93,95]. Thus, to predict individual responses to anti-TNFα agents, genetic variants involved in immune processes, inflammation, autophagy, and apoptosis have been investigated [10].
In a recent genome-wide study including 1,240 biologic-naïve patients with CD, the HLA-DQA1*05 allele, carried by 40% of Europeans, was identified as a genetic determinant of immunogenicity. Carriership of the HLA-DQA1*05 haplotype increased the immunogenicity regardless of medication (IFX; hazard ratio [HR], 1.92; 95% CI, 1.57 to 2.33) (adalimumab; HR, 1.89; 95% CI, 1.32 to 2.70) and concomitant use of an immunosuppressive agent (anti-TNFα monotherapy [HR, 1.75; 95% CI, 1.37 to 2.22], combination therapy [HR, 2.01; 95% CI, 1.57 to 2.58]) [96]. Another cohort study including 262 IBD patients found that the HLA-DQA1*05 variant increased not only the risk of IFX antibody formation (adjusted HR, 7.29; 95% CI, 2.97 to 17.191; P = 1.46 × 10-5) independent of age, sex, weight, dose, and concomitant use of immunomodulatory, but also the risk of loss of response (adjusted HR, 2.34; 95% CI, 1.41 to 3.88; P= 0.001) and discontinuation (adjusted HR, 2.27; 95% CI, 1.46 to 3.43; P= 2.53× 10-4) [97].
Several candidate genes to predict treatment response based on the pathogenesis of IBD or mechanisms of action of anti-TNFα have also been suggested. In 2014, Bank et al. [98] reported that 19 out of 39 functional polymorphisms in 26 genes that alter the NFκB-mediated inflammatory response (TLR2 [rs3804099, rs11938228, rs1816702, rs469 6480], TLR4 [rs5030728, rs1554973], TLR9 [rs187084, rs352 139], LY96 [MD-2] [rs11465996], CD14 [rs2569190], MAP3K14 [NIK] [rs7222094]), TNFα signaling (TNFA [TNFα] [rs361525], TNFRSF1A [TNFR1] [rs4149570], TNFAIP3 [A20] [rs6927172]), and other cytokines regulated by NFκB (IL1B [rs4848306], IL1RN [rs4251961], IL6 [rs10499563], IL17A [rs2275913], IFNG [rs2430561]) other cytokines regulated by NFκB (IL1B [rs484 8306], IL1RN [rs4251961], IL6 [rs10499563], IL17A [rs227 5913], IFNG [rs2430561]) were associated with response to anti-TNFα therapy in 738 anti-TNFα-naïve IBD patients [98]. In 2019, they replicated and updated the gene signature in a newly established IBD cohort. Ten polymorphisms in genes involved in the regulation of NFκB pathways (TLR2, TLR4, and NFKBIA), TNFα signaling pathway (TNFRSF1A), and cytokine pathways (NLRP3, IL1RN, IL18, and JAK2) were associated with response to anti-TNFα therapy [99].
With extreme phenotype approach that identified patients at opposite ends of IFX response, rs2158962 in TNF receptor associated protein 1 (TRAP1) was found to have a high rate of association with mucosal healing in CD patients (OR, 4.94; Pcombined=1.35×10−7). When the predictive power of rs2158962 was assessed in beyond extreme cases, mucosal healing rate was 5.5-fold higher in homozygotes (P= 6.10× 10−5), and 2-fold higher in heterozygotes (P= 0.024) compared with that in non-carriers. In the dextran sodium sulfate-induced acute colitis mice model, TRAP1 transgenic mice also exhibited a better response in histologic recovery compared with the wild-type mice [12].
Genetic analysis combined with clinical characteristics have been attempted to improve the predictive power of the response to anti-TNFα therapy. Recently, a Korean-German study team identified the clinical and genetic markers involved in IFX response using whole-exome sequencing data. The rs 2228273 in zinc finger protein 133 (ZNF133) (OR, 11.94; P= 2.10×10−5), concurrent azathioprine/6-mercaptopurine use (OR, 4.78; P=0.031), and bodyweight under 50 kg at the first IFX use (OR, 5.26; P = 0.013) were associated with primary non-response. The combined genetic and clinical models had superior predictive power compared to the model including only genetic variables or only clinical variables (area under the receiver operating characteristics [AUROCs], 0.84, 0.70, and 0.69, respectively) [100]. ZNF133 is a Krüppel-associated box-zinc finger family protein [100] and is known to repress S100A4 gene expression [101]. High levels of S100A4, a member of the S100 family of calcium-binding proteins, are associated with a poor clinical IFX response and high occurrence of anti-IFX antibodies in rheumatoid arthritis patients [101].
In a prospective study comprising 231 UC patients, a combined clinical-genetic model and clinical predictive model were compared for primary nonresponse and durable response. For genotyping, a platform with 196,524 polymorphisms (718 small insertion deletions, 195,806 SNPs) of known major immune and inflammatory disease loci was used. With detection of 8 alleles associated with primary nonresponse (potential genes PTPN22, PHTF1, TRAF3IP2-AS1, NFIL3, FIBP, SH2B3, ATXN2, UBAC2, GPR18, IFNGR2, IFNAR1, IL10RB), the combined clinical-genetic model showed higher power than that in the clinical-only model (AUROCs, 0.87 vs. 0.57, P< 0.0001). Interestingly, the identified SNPs involved in primary nonresponse were not associated with IFX antibodies or serum drug levels, which implies that the mechanisms of these genetic loci might be mediated by other than drug pharmacokinetics or antibody formation [102]. Moreover, a Dutch study team recently explored the efficacy of pharmacogenetic passport, including the genetic variation of TPMT, NUDT15, HLA-DQA1*02:01-HLA-DRB1*07:01 (for thiopurine-induced pancreatitis), and HLA-DQA1*05. The pharmacogenetic passport predicted 36% of thiopurine or anti-TNFα adverse responses in 150 cases. The calculated numbers needed to genotype, treat, and harm to prevent one of these adverse drug responses were 24, 9, and 6, respectively. With the estimation of the numbers needed for genotyping, 24 patients should undergo pretreatment genotyping for prevention. This means that if pretreatment genotyping is performed in 24 patients, one adverse drug response would be prevented using an appropriate alternative treatment strategy [103].
Personalized medicine based on individual pharmacogenetics is necessary to improve clinical outcomes in the management of IBD, which requires long-term treatment. In addition to efficacy and safety issues, personalized therapy would be beneficial in reducing the treatment cost. They could optimize safe and cheap conventional treatment, avoid expensive but ineffective or harmful drugs, especially cost-associated anti-TNFα adverse reactions, and achieve optimal dosing during an early treatment period [9].
However, pharmacogenetics-based IBD treatment in the clinical setting is still challenging, with only several genetic predictive factors for thiopurines being validated and recommended by guidelines (Fig. 2). Studies on other drugs have shown inconsistent results, or include relatively small sample cohorts. To overcome these limitations, future studies involving larger nationwide and international collaborative designs should be conducted and should evaluate combined epigenetic, environmental and clinical features as well as genetic biomarkers [10]. With the development of relevant markers, tools for interpreting genetic information for appropriate clinical implementation could be provided [10].
Notes
AUTHOR CONTRIBUTIONS
Conception or design: JYC, JHC.
Acquisition, analysis, or interpretation of data: JYC.
Drafting the work or revising: JYC.
Final approval of the manuscript: JYC, JHC.
Fig. 1.
Guideline for optimal prescription of thiopurine. CBC, complete blood count; LFT, liver function test; NUDT, nudix hydrolase; FTO, fat mass and obesity-associated; TPMT, thiopurine S-methyltransferase; HLA, human leukocyte antigen.
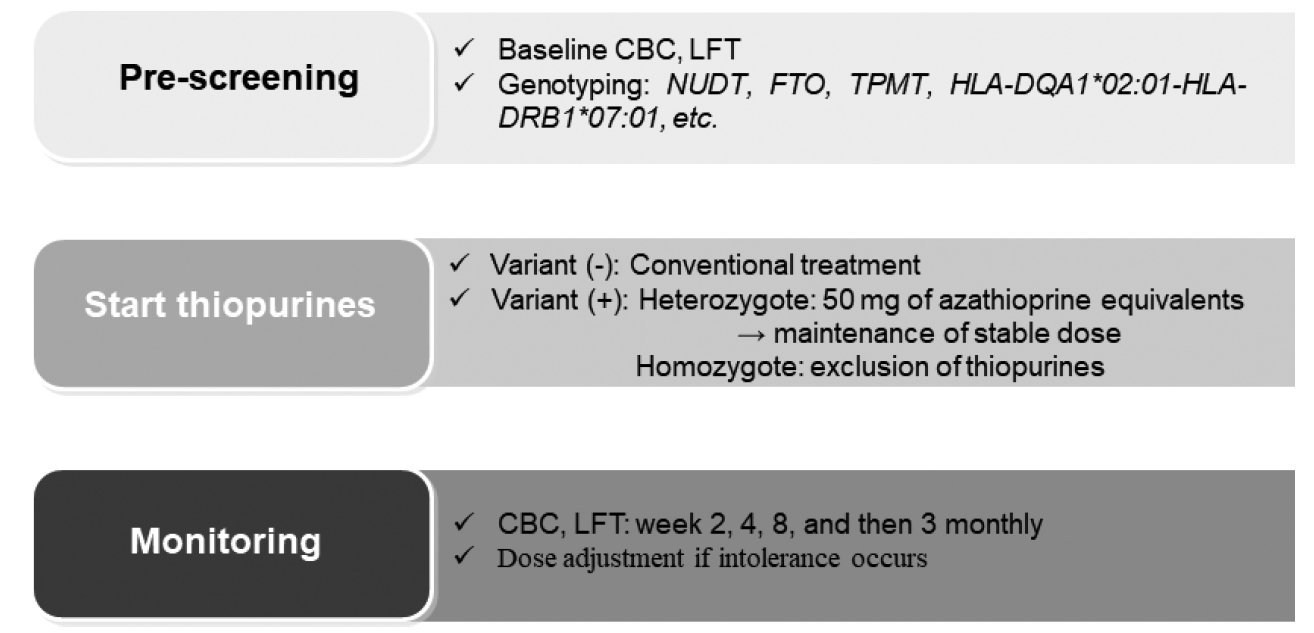
Fig. 2.
Proposed personalized treatment of inflammatory bowel disease based on clinical and pharmacogenetic factors. NUDT15, nudix hydrolase 15; FTO, fat mass and obesity-associated; TPMT, thiopurine S-methyltransferase; HLA, human leukocyte antigen; ZNF133, zinc finger protein 133; RGS17, regulator of G-protein signaling 17.
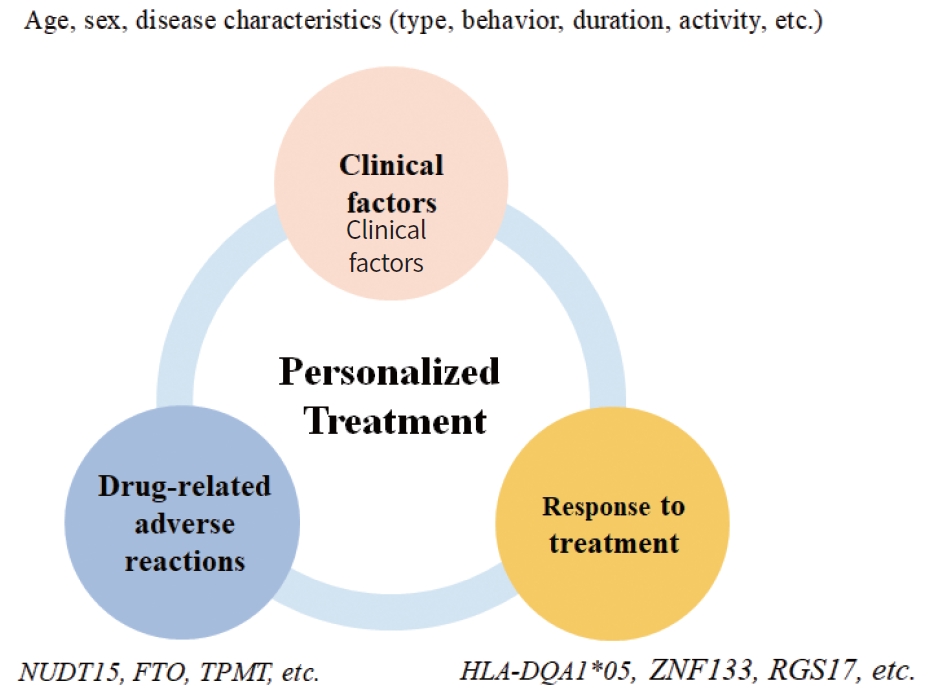
Table 1.
Pharmacogenetic candidates in the management of inflammatory bowel disease
REFERENCES
1. Yamamoto-Furusho JK. Pharmacogenetics in inflammatory bowel disease: understanding treatment response and personalizing therapeutic strategies. Pharmgenomics Pers Med 2017;10:197–204.



2. Mizoguchi E, Low D, Ezaki Y, Okada T. Recent updates on the basic mechanisms and pathogenesis of inflammatory bowel diseases in experimental animal models. Intest Res 2020;18:151–67.



3. Hong SW, Ye BD. The first step to unveil the epidemiology of inflammatory bowel disease in Central Asia. Intest Res 2020;18:345–6.



4. Carter MJ, Lobo AJ, Travis SP, IBD Section; British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut 2004;53(Suppl 5):V1–16.



5. Yagisawa K, Kobayashi T, Ozaki R, Okabayashi S, Toyonaga T, Miura M, et al. Randomized, crossover questionnaire survey of acceptabilities of controlled-release mesalazine tablets and granules in ulcerative colitis patients. Intest Res 2019;17:87–93.


6. Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis 2012;6:965–90.


7. Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, Fidder H, et al. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis 2011;5:477–83.


8. Shimizu H, Suzuki K, Watanabe M, Okamoto R. Stem cellbased therapy for inflammatory bowel disease. Intest Res 2019;17:311–6.



9. Voskuil MD, Bangma A, Weersma RK, Festen EA. Predicting (side) effects for patients with inflammatory bowel disease: the promise of pharmacogenetics. World J Gastroenterol 2019;25:2539–48.



10. Lucafo M, Franca R, Selvestrel D, Curci D, Pugnetti L, Decorti G, et al. Pharmacogenetics of treatments for inflammatory bowel disease. Expert Opin Drug Metab Toxicol 2018;14:1209–23.


11. Lee HS, Cleynen I. Molecular profiling of inflammatory bowel disease: is it ready for use in clinical decision-making? Cells 2019;8:535.



12. Park SH, Hong M, Lee HS, Ye BD, Hwang SW, Jung S, et al. Association of TRAP1 with infliximab-induced mucosal healing in Crohn’s disease. J Gastroenterol Hepatol 2019;34:2118–25.



13. Perez-Gracia JL, Gurpide A, Ruiz-Ilundain MG, Alfaro Alegria C, Colomer R, Garcia-Foncillas J, et al. Selection of extreme phenotypes: the role of clinical observation in translational research. Clin Transl Oncol 2010;12:174–80.



14. Perez-Gracia JL, Sanmamed MF, Bosch A, Patino-Garcia A, Schalper KA, Segura V, et al. Strategies to design clinical studies to identify predictive biomarkers in cancer research. Cancer Treat Rev 2017;53:79–97.


15. Sood A, Ahuja V, Midha V, Sinha SK, Pai CG, Kedia S, et al. Colitis and Crohn’s Foundation (India) consensus statements on use of 5-aminosalicylic acid in inflammatory bowel disease. Intest Res 2020;18:355–78.



16. Mizuno S, Ono K, Mikami Y, Naganuma M, Fukuda T, Minami K, et al. 5-Aminosalicylic acid intolerance is associated with a risk of adverse clinical outcomes and dysbiosis in patients with ulcerative colitis. Intest Res 2020;18:69–78.



17. Lichtenstein GR, Kamm MA. Review article: 5-aminosalicylate formulations for the treatment of ulcerative colitis: methods of comparing release rates and delivery of 5-aminosalicylate to the colonic mucosa. Aliment Pharmacol Ther 2008;28:663–73.


18. Yamamoto-Furusho JK, Fonseca-Camarillo G. Genetic markers associated with clinical outcomes in patients with inflammatory bowel disease. Inflamm Bowel Dis 2015;21:2683–95.


19. Hickman D, Pope J, Patil SD, Fakis G, Smelt V, Stanley LA, et al. Expression of arylamine N-acetyltransferase in human intestine. Gut 1998;42:402–9.



20. Das KM, Eastwood MA, McManus JP, Sircus W. Adverse reactions during salicylazosulfapyridine therapy and the relation with drug metabolism and acetylator phenotype. N Engl J Med 1973;289:491–5.


21. Hausmann M, Paul G, Menzel K, Brunner-Ploss R, Falk W, Scholmerich J, et al. NAT1 genotypes do not predict response to mesalamine in patients with ulcerative colitis. Z Gastroenterol 2008;46:259–65.


22. Ricart E, Taylor WR, Loftus EV, O’Kane D, Weinshilboum RM, Tremaine WJ, et al. N-acetyltransferase 1 and 2 genotypes do not predict response or toxicity to treatment with mesalamine and sulfasalazine in patients with ulcerative colitis. Am J Gastroenterol 2002;97:1763–8.


23. Olaisen M, Spigset O, Flatberg A, Granlund AV, Brede WR, Albrektsen G, et al. Mucosal 5-aminosalicylic acid concentration, drug formulation and mucosal microbiome in patients with quiescent ulcerative colitis. Aliment Pharmacol Ther 2019;49:1301–13.



24. Yamamoto-Furusho JK, Jacintez-Cazares M, Furuzawa-Carballeda J, Fonseca-Camarillo G. Peroxisome proliferator-activated receptors family is involved in the response to treatment and mild clinical course in patients with ulcerative colitis. Dis Markers 2014;2014:932530.



25. Yamamoto-Furusho JK, Penaloza-Coronel A, Sanchez-Munoz F, Barreto-Zuniga R, Dominguez-Lopez A. Peroxisome proliferator-activated receptor-gamma (PPAR-γ) expression is downregulated in patients with active ulcerative colitis. Inflamm Bowel Dis 2011;17:680–1.


26. Heap GA, So K, Weedon M, Edney N, Bewshea C, Singh A, et al. Clinical features and HLA association of 5-aminosalicylate (5-ASA)-induced nephrotoxicity in inflammatory bowel disease. J Crohns Colitis 2016;10:149–58.


27. Matsumoto S, Mashima H. Mesalazine allergy and an attempt at desensitization therapy in patients with inflammatory bowel disease. Sci Rep 2020;10:22176.



28. Saito D, Hayashida M, Sato T, Minowa S, Ikezaki O, Mitsui T, et al. Evaluation of the drug-induced lymphocyte stimulation test for diagnosing mesalazine allergy. Intest Res 2018;16:273–81.



29. Suzuki K, Kakuta Y, Naito T, Takagawa T, Hanai H, Araki H, et al. Genetic background of mesalamine-induced fever and diarrhea in Japanese patients with inflammatory bowel disease. Inflamm Bowel Dis 2021 Jan 27 [Epub]. https://doi.org/10.1093/ibd/izab004.

30. Hayes MP, Roman DL. Regulator of G protein signaling 17 as a negative modulator of GPCR signaling in multiple human cancers. AAPS J 2016;18:550–9.



31. Skrzypczak-Zielinska M, Gabryel M, Marszalek D, Dobrowolska A, Slomski R. NGS study of glucocorticoid response genes in inflammatory bowel disease patients. Arch Med Sci 2019;17:417–33.



32. Farrell RJ, Kelleher D. Glucocorticoid resistance in inflammatory bowel disease. J Endocrinol 2003;178:339–46.


33. Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a populationbased study. Gastroenterology 2001;121:255–60.


34. De Iudicibus S, Franca R, Martelossi S, Ventura A, Decorti G. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J Gastroenterol 2011;17:1095–108.



35. Mahajan R, Singh A, Kedia S, Kaur K, Midha V, Sahu P, et al. Maintaining infliximab induced clinical remission with azathioprine and 5-aminosalicylates in acute severe steroid-refractory ulcerative colitis has lower cost and high efficacy (MIRACLE): a multicenter study. Intest Res 2021 Feb 3 [Epub]. https://doi.org/10.5217/ir.2020.00100.

36. Raddatz D, Middel P, Bockemuhl M, Benohr P, Wissmann C, Schworer H, et al. Glucocorticoid receptor expression in inflammatory bowel disease: evidence for a mucosal down-regulation in steroid-unresponsive ulcerative colitis. Aliment Pharmacol Ther 2004;19:47–61.


37. De Iudicibus S, Stocco G, Martelossi S, Drigo I, Norbedo S, Lionetti P, et al. Association of BclI polymorphism of the glucocorticoid receptor gene locus with response to glucocorticoids in inflammatory bowel disease. Gut 2007;56:1319–20.

38. van Rossum EF, Koper JW, van den Beld AW, Uitterlinden AG, Arp P, Ester W, et al. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol (Oxf) 2003;59:585–92.


39. Huizenga NA, Koper JW, De Lange P, Pols HA, Stolk RP, Burger H, et al. A polymorphism in the glucocorticoid receptor gene may be associated with and increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab 1998;83:144–51.

40. Chen HL, Li LR. Glucocorticoid receptor gene polymorphisms and glucocorticoid resistance in inflammatory bowel disease: a meta-analysis. Dig Dis Sci 2012;57:3065–75.


41. Ahmad T, Tamboli CP, Jewell D, Colombel JF. Clinical relevance of advances in genetics and pharmacogenetics of IBD. Gastroenterology 2004;126:1533–49.


42. Palmieri O, Latiano A, Valvano R, D’Inca R, Vecchi M, Sturniolo GC, et al. Multidrug resistance 1 gene polymorphisms are not associated with inflammatory bowel disease and response to therapy in Italian patients. Aliment Pharmacol Ther 2005;22:1129–38.


43. Croucher PJ, Mascheretti S, Foelsch UR, Hampe J, Schreiber S. Lack of association between the C3435T MDR1 gene polymorphism and inflammatory bowel disease in two independent Northern European populations. Gastroenterology 2003;125:1919–20.


44. Ho GT, Nimmo ER, Tenesa A, Fennell J, Drummond H, Mowat C, et al. Allelic variations of the multidrug resistance gene determine susceptibility and disease behavior in ulcerative colitis. Gastroenterology 2005;128:288–96.


45. McGovern D, Ahmad T, van Heel D, Negoro K, Jewell D. Cytochrome P450 and multidrug-resistance gene 1 (MDR-1) polymorphisms: predictors of the need for colectomy in ulcerative colitis? Gastroenterology 2002;122(Suppl 4):A607.
46. Potocnik U, Ferkolj I, Glavac D, Dean M. Polymorphisms in multidrug resistance 1 (MDR1) gene are associated with refractory Crohn disease and ulcerative colitis. Genes Immun 2004;5:530–9.


47. Miller AH, Pariante CM, Pearce BD. Effects of cytokines on glucocorticoid receptor expression and function: glucocorticoid resistance and relevance to depression. Adv Exp Med Biol 1999;461:107–16.

48. Louis E, Peeters M, Franchimont D, Seidel L, Fontaine F, Demolin G, et al. Tumour necrosis factor (TNF) gene polymorphism in Crohn’s disease (CD): influence on disease behaviour? Clin Exp Immunol 2000;119:64–8.



49. De Iudicibus S, Stocco G, Martelossi S, Londero M, Ebner E, Pontillo A, et al. Genetic predictors of glucocorticoid response in pediatric patients with inflammatory bowel diseases. J Clin Gastroenterol 2011;45:e1–7.


50. Timmer A, Patton PH, Chande N, McDonald JW, MacDonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2016;2016:CD000478.



51. Lichtenstein GR, Abreu MT, Cohen R, Tremaine W, American Gastroenterological Association. American Gastroenterological Association institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006;130:940–87.


52. Lee KM, Kim YS, Seo GS, Kim TO, Yang SK, IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Use of thiopurines in inflammatory bowel disease: a consensus statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res 2015;13:193–207.



53. Panaccione R, Ghosh S, Middleton S, Marquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392–400.


54. Chang JY, Cheon JH. Thiopurine therapy in patients with inflammatory bowel disease: a focus on metabolism and pharmacogenetics. Dig Dis Sci 2019;64:2395–403.


55. Chaparro M, Ordas I, Cabre E, Garcia-Sanchez V, Bastida G, Penalva M, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis 2013;19:1404–10.

56. Gisbert JP, Gomollon F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol 2008;103:1783–800.


57. Gearry RB, Barclay ML, Burt MJ, Collett JA, Chapman BA. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf 2004;13:563–7.


58. Warman JI, Korelitz BI, Fleisher MR, Janardhanam R. Cumulative experience with short- and long-term toxicity to 6-mercaptopurine in the treatment of Crohn’s disease and ulcerative colitis. J Clin Gastroenterol 2003;37:220–5.


59. Lee HJ, Yang SK, Kim KJ, Choe JW, Yoon SM, Ye BD, et al. The safety and efficacy of azathioprine and 6-mercaptopurine in the treatment of Korean patients with Crohn’s disease. Intest Res 2009;7:22–31.
60. Kim JH, Cheon JH, Hong SS, Eun CS, Byeon JS, Hong SY, et al. Influences of thiopurine methyltransferase genotype and activity on thiopurine-induced leukopenia in Korean patients with inflammatory bowel disease: a retrospective cohort study. J Clin Gastroenterol 2010;44:e242–8.

61. Qiu Y, Mao R, Zhang SH, Li MY, Guo J, Chen BL, et al. Safety profile of thiopurines in Crohn disease: analysis of 893 patient-years follow-up in a Southern China cohort. Medicine (Baltimore) 2015;94:e1513.


62. Odahara S, Uchiyama K, Kubota T, Ito Z, Takami S, Kobayashi H, et al. A prospective study evaluating metabolic capacity of thiopurine and associated adverse reactions in Japanese patients with inflammatory bowel disease (IBD). PLoS One 2015;10:e0137798.



63. Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut 2002;50:485–9.



64. Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut 1993;34:1081–5.



65. Watanabe A, Hobara N, Nagashima H. Demonstration of enzymatic activity converting azathioprine to 6-mercaptopurine. Acta Med Okayama 1978;32:173–9.

66. Zaza G, Cheok M, Krynetskaia N, Thorn C, Stocco G, Hebert JM, et al. Thiopurine pathway. Pharmacogenet Genomics 2010;20:573–4.



67. Collie-Duguid ES, Pritchard SC, Powrie RH, Sludden J, Collier DA, Li T, et al. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics 1999;9:37–42.


68. Katsanos KH, Tsianos EV. Azathioprine/6-mercaptopurine toxicity: the role of the TPMT gene. Ann Gastroenterol 2007;20:251–64.
69. Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet 2014;46:1017–20.



70. Kham SK, Soh CK, Liu TC, Chan YH, Ariffin H, Tan PL, et al. Thiopurine S-methyltransferase activity in three major Asian populations: a population-based study in Singapore. Eur J Clin Pharmacol 2008;64:373–9.


71. Dewit O, Moreels T, Baert F, Peeters H, Reenaers C, de Vos M, et al. Limitations of extensive TPMT genotyping in the management of azathioprine-induced myelosuppression in IBD patients. Clin Biochem 2011;44:1062–6.


72. Ansari A, Arenas M, Greenfield SM, Morris D, Lindsay J, Gilshenan K, et al. Prospective evaluation of the pharmacogenetics of azathioprine in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther 2008;28:973–83.


73. Ooi CJ, Hilmi I, Banerjee R, Chuah SW, Ng SC, Wei SC, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn’s disease in Asia. Intest Res 2019;17:285–310.



74. Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet 2016;48:367–73.



75. Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol 2015;33:1235–42.



76. Matsuoka K. NUDT15 gene variants and thiopurine-induced leukopenia in patients with inflammatory bowel disease. Intest Res 2020;18:275–81.



77. Kim HS, Cheon JH, Jung ES, Park J, Aum S, Park SJ, et al. A coding variant in FTO confers susceptibility to thiopurine-induced leukopenia in East Asian patients with IBD. Gut 2017;66:1926–35.


78. Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer 2008;8:24–36.


79. Fedeles BI, Singh V, Delaney JC, Li D, Essigmann JM. The AlkB family of Fe(II)/α-ketoglutarate-dependent dioxygenases: repairing nucleic acid alkylation damage and beyond. J Biol Chem 2015;290:20734–42.



80. Chang JY, Park SJ, Jung ES, Jung SA, Moon CM, Chun J, et al. Genotype-based treatment with thiopurine reduces incidence of myelosuppression in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2020;18:2010–8.


81. Wilson A, Jansen LE, Rose RV, Gregor JC, Ponich T, Chande N, et al. HLA-DQA1-HLA-DRB1 polymorphism is a major predictor of azathioprine-induced pancreatitis in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2018;47:615–20.


82. Teich N, Mohl W, Bokemeyer B, Bundgens B, Buning J, Miehlke S, et al. Azathioprine-induced acute pancreatitis in patients with inflammatory bowel diseases: a prospective study on incidence and severity. J Crohns Colitis 2016;10:61–8.


83. Heap GA, Weedon MN, Bewshea CM, Singh A, Chen M, Satchwell JB, et al. HLA-DQA1-HLA-DRB1 variants confer susceptibility to pancreatitis induced by thiopurine immunosuppressants. Nat Genet 2014;46:1131–4.



84. Dai C, Liu WX, Jiang M, Sun MJ. Mucosal healing did not predict sustained clinical remission in patients with IBD after discontinuation of one-year infliximab therapy. PLoS One 2014;9:e110797.



85. Shin SY, Park SJ, Kim Y, Im JP, Kim HJ, Lee KM, et al. Clinical outcomes and predictors of response for adalimumab in patients with moderately to severely active ulcerative colitis: a KASID prospective multicenter cohort study. Intest Res 2021;Jul 23 [Epub]. https://doi.org/10.5217/ir.2021.00049.

86. Hisamatsu T, Suzuki Y, Kobayashi M, Hagiwara T, Kawaberi T, Ogata H, et al. Long-term safety and effectiveness of adalimumab in Japanese patients with Crohn’s disease: 3-year results from a real-world study. Intest Res 2021;19:408–18.


87. Oh SJ, Shin GY, Soh H, Lee JG, Im JP, Eun CS, et al. Longterm outcomes of infliximab in a real-world multicenter cohort of patients with acute severe ulcerative colitis. Intest Res 2021;19:323–31.


88. Nakamura S, Asano T, Tsuchiya H, Sugimoto K, Imai Y, Yokoyama S, et al. Real-world data for golimumab treatment in patients with ulcerative colitis in Japan: interim analysis in post-marketing surveillance. Intest Res 2021;Aug 4 [Epub]. https://doi.org/10.5217/ir.2021.00032.

89. Moroi R, Endo K, Yamamoto K, Naito T, Onodera M, Kuroha M, et al. Long-term prognosis of Japanese patients with biologic-naive Crohn’s disease treated with anti-tumor necrosis factor-α antibodies. Intest Res 2019;17:94–106.


90. Allez M, Karmiris K, Louis E, Van Assche G, Ben-Horin S, Klein A, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4:355–66.


91. Fukuda T, Naganuma M, Kanai T. Current new challenges in the management of ulcerative colitis. Intest Res 2019;17:36–44.



92. Sood A, Singh A, Sudhakar R, Midha V, Mahajan R, Mehta V, et al. Exclusive enteral nutrition for induction of remission in anti-tumor necrosis factor refractory adult Crohn’s disease: the Indian experience. Intest Res 2020;18:184–91.



93. Vermeire S, Gils A, Accossato P, Lula S, Marren A. Immunogenicity of biologics in inflammatory bowel disease. Therap Adv Gastroenterol 2018;11:1756283X17750355.



94. Theodoraki E, Orfanoudaki E, Foteinogiannopoulou K, Legaki E, Gazouli M, Koutroubakis IE. Is there a correlation between infliximab trough levels and the development of adverse events in patients with inflammatory bowel disease? Intest Res 2021;19:461–7.


95. Singh A, Mahajan R, Kedia S, Dutta AK, Anand A, Bernstein CN, et al. Use of thiopurines in inflammatory bowel disease: an update. Intest Res 2021;Apr 15 [Epub]. https://doi.org/10.5217/ir.2020.00155.

96. Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology 2020;158:189–99.

97. Wilson A, Peel C, Wang Q, Pananos AD, Kim RB. HLADQA1*05 genotype predicts anti-drug antibody formation and loss of response during infliximab therapy for inflammatory bowel disease. Aliment Pharmacol Ther 2020;51:356–63.


98. Bank S, Andersen PS, Burisch J, Pedersen N, Roug S, Galsgaard J, et al. Associations between functional polymorphisms in the NFκB signaling pathway and response to anti-TNF treatment in Danish patients with inflammatory bowel disease. Pharmacogenomics J 2014;14:526–34.


99. Bank S, Julsgaard M, Abed OK, Burisch J, Broder Brodersen J, Pedersen NK, et al. Polymorphisms in the NFkB, TNF-alpha, IL-1beta, and IL-18 pathways are associated with response to anti-TNF therapy in Danish patients with inflammatory bowel disease. Aliment Pharmacol Ther 2019;49:890–903.


100. Jung ES, Choi KW, Kim SW, Hubenthal M, Mucha S, Park J, et al. ZNF133 is associated with infliximab responsiveness in patients with inflammatory bowel diseases. J Gastroenterol Hepatol 2019;34:1727–35.



101. Erlandsson MC, Forslind K, Andersson SE, Lund A, Bokarewa MI. Metastasin S100A4 is increased in proportion to radiographic damage in patients with RA. Rheumatology (Oxford) 2012;51:932–40.









