 |
 |
- Search
| Precis Future Med > Volume 7(2); 2023 > Article |
|
Abstract
Purpose
Among patients with acute ischemic stroke (AIS), those with intracranial large vessel occlusion (LVO) should undertake endovascular treatment (EVT) based on mechanical thrombectomy. Although the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system has been used in overall population of patients with AIS, especially for secondary prevention. In the current study, a new classification system for the LVO population is proposed.
Methods
The classic TOAST and Stop Stroke Study TOAST (SSS TOAST) were applied to the LVO population. Based on discordance with those systems, a new LVO classification system was developed and applied to the LVO population. The new system comprised extracranial atherosclerosis (ECAS), intracranial atherosclerosis (ICAS), cardioembolism (CE), cryptogenic embolism, stroke of undetermined etiology (SUE; two or more etiologies), and stroke of other determined etiology (SOE) where small artery occlusion was removed.
Results
The LVO classification system comprised 43 ECAS (6.52%), 141 ICAS (21.36%), 303 CE (45.91%), 75 cryptogenic embolism (11.36%), 75 SUE (11.36%; cardioembolic source in 98.67%), and 23 SOE (3.48%) patients. The ICAS group had a significantly longer median onset-to-puncture time than the other groups. In the ICAS group, 102 of 141 (72.34%) remained partial recanalization after EVT.
Conclusion
The LVO classification system differentiating ECAS and ICAS in patients with large artery atherosclerosis and classifying cryptogenic embolism is more suitable for patients with EVT for intracranial LVO. Further studies for predicting underlying ICAS and planning treatment strategy should be performed.
Appropriate classification of the causative mechanism of stroke is important for optimizing treatment and assessing prognosis. The Trial of Org 10172 in Acute Stroke Treatment (TOAST) and Stop Stroke Study TOAST (SSS TOAST) are two main stroke classification systems, and their main purpose is secondary prevention [1,2]. With the progress of testing techniques for understanding the mechanism of ischemic stroke, several stroke classification systems based on etiologic mechanisms have been proposed. However, despite the emergence of newer classification systems, the older TOAST and SSS TOAST classifications remain the most widely utilized due to their simplicity and widespread adoption. Proper classification of strokes based on their underlying causative mechanisms is essential for guiding treatment decisions, predicting prognosis, and evaluating the effectiveness of interventions in clinical trials [3].
Endovascular treatment (EVT) is now widely used in patients with acute ischemic stroke (AIS) due to intracranial large vessel occlusion (LVO) [4]. As EVT has emerged as a pivotal treatment for patients with ischemic stroke, there is an increasing demand for updated stroke classifications or improvements to existing systems like TOAST and SSS TOAST [5]. These advancements are essential for enabling precise prognostic assessment and facilitating future clinical trials in this specific patient population. On the other hand, most randomized control studies on EVT have focused on LVO so that the optimal treatment approach for extracranial internal carotid artery (ICA) lesions remains uncertain. In case of the acute phase EVT for a tandem occlusion, it may be necessary to consider isolated EVT for intracranial LVO or also plan stenting for extracranial ICA stenosis [6]. In this case, the patient has atherosclerotic disease on extracranial ICA whereas there is an embolism on the intracranial vessel. Therefore, there is a need for a classification system that can guide future treatment decisions and facilitate prognostic comparisons in these scenarios.
Herein, we applied the classic and SSS TOAST systems whether these systems are fit for LVO population. Based on discordance among them, we propose a novel LVO classification system for patients undergoing EVT with a pilot study nature.
The Institutional Review Board of Ajou University Hospital in Suwon, Korea approved this retrospective, single center, observational study, which was conducted in accordance with the Declaration of Helsinki (AJOUIRB-MDB-2021-664). The requirement for informed consent was waived due to the retrospective nature of the research.
This retrospective, single center, observational study included ischemic stroke patients whose data were obtained from an institutional stroke registry in Ajou University Hospital and who received emergent EVT between January 2011 and May 2021. Among the cohort, a subset of 660 LVO patients who underwent EVT were selected and included in the study.
For the stroke index, patients were subjected to a three-axis etiological workup [7]. For the vessel axis, large arterial diseases, such as atherosclerosis and dissection, were determined by the final angiography of EVT and post-EVT short-term repeat computed tomographic angiography [8]. High-resolution vessel wall magnetic resonance imaging (MRI) was performed, especially for patients with suspected intracranial dissection. For the cardioembolic axis, electrocardiography and echocardiogram were performed. When there was suspicion of stroke caused by cardioembolism (CE), we conducted Holter monitoring, while transesophageal echocardiography was performed in certain cases. For hematological axis, various laboratory tests, including D-dimer, fibrinogen degradation product, fibrinogen, protein C, protein S, antinuclear antibody, anti-cardiolipin antibody, and antithrombin III, were performed. Following EVT, we classified cases with modified Thrombolysis in Cerebral Infarction (mTICI) grade 0–1 as intractable. Cases with mTICI grade 2a were classified as partial recanalization, indicating the presence of incomplete reperfusion [9].
LVO patients were classified according to the TOAST and SSS TOAST. As a control, the classic TOAST-applied data for overall patients with AIS (with or without LVO) were retrieved from a previous report utilizing the Korean Stroke Registry [10]. According to the classic TOAST, they were classified as large artery atherosclerosis (LAA), CE, small artery occlusion (SAO), stroke of other determined etiology (SOE), and stroke of undetermined etiology (SUE) [1]. Furthermore, as per the SSS TOAST, patients were classified as LAA, CE, SAO, cryptogenic, SOE, and SUE (having > 1 etiology) [2].
A new LVO classification was based on the discordance of the classic and SSS TOAST classifications with LVO and EVT patients. In this classification, the LAA group by SSS TOAST was further classified into extracranial atherosclerosis (ECAS) and intracranial atherosclerosis (ICAS) groups. For posterior circulation infarction attributed to LAA, we classified ECAS involving up to the V3 segment of the vertebral artery, and ICAS involving the V4 segment up to the basilar artery. In the SSS TOAST classification system, SUEs in the classic TOAST are categorized as either cryptogenic embolism or SUE with two or more potential etiologies. However, in our study, we further differentiated between cryptogenic embolisms and cases with multiple etiologies within the SUE category. Therefore, cases with no identified etiology were classified as cryptogenic embolism and cases with multiple etiologies were classified as SUE in our study. SAO groups were excluded from this reclassification process.
Baseline demographics, initial severity, and outcomes were analyzed for each classification. Continuous variables were presented as mean± standard deviation, median (interquartile range), or number (percentage) as appropriate. To compare the two groups, the Student’s t-test and Mann-Whitney U-test were used for continuous variables, while the chisquare test was used for categorical variables. For comparing three groups, one-way analysis of variance (ANOVA) and post hoc Tamhane’s test were used for continuous variables, and the chi-square test was used for categorical variables. Statistical significance was set at P< 0.05. All statistical analyses were performed using the R Statistical Software version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Of the 660 enrolled patients with LVO and EVT, the classic TOAST system comprised 172 LAA (26.06%), 302 CE (45.76%), 0 SAO (0%), 163 SUE (24.70%), and 23 SOE (3.48%) patients. The SSS TOAST system comprised 184 LAA (27.88%), 303 CE (45.91%), 0 SAO (0%), 75 cryptogenic embolism (11.36%), 75 SUE (11.36%; cardioembolic source in 98.67%), and 23 SOE (3.48%) patients. In contrast, based on the Korean Stroke Registry during 2014 to 2018, overall patients with AIS with or without LVO comprised LAA (32.6%), CE (21.3%), SAO (19.9%), SUE (22.5%), and SOE (3.7%) in the classic TOAST system [11]. The proportions of etiologic subgroups of LVO patients compared with overall stroke patients from the Korean Stroke Registry are illustrated in Fig. 1.
In the new LVO classification system, patients classified as having LAA by the SSS TOAST were further classified into ECAS and ICAS (Table 1). The LVO classification system comprised 43 ECAS (6.52%), 141 ICAS (21.36%), 303 CE (45.91%), 75 cryptogenic embolism (11.36%), 75 SUE (11.36%; cardioembolic source in 98.67%), and 23 SOE (3.48%) patients. A total of 10 of 43 (23.36%) and 102 of 141 (72.34%) ECAS and ICAS patients, respectively, remained partial recanalization after EVT for treating with intracranial LVO (P < 0.001) (Table 1). The ICAS group had a significantly longer median onset-to-puncture time than the other groups, including the ECAS group (P < 0.001). The proportions of etiological subgroups of LVO patients were applied to the new classification system (Fig. 2). The advantages and limitations or suggestions comparing the classic and SSS TOAST and the new LVO etiological classification systems are described in Table 2.
There was discordance in TOAST classifications between the overall Korean population with AIS in previous studies [3,10] and the LVO population that received EVT in our study. CE was a major etiology in our study population, whereas the major etiology was LAA in the overall Korean population with AIS [3,10]. Emergent LVO patients represent only 10% to 20% of all AIS patients, but they require their own classification for acute treatment planning and clinical outcome prediction. No SAO patient was included in the LVO population from both TOAST systems. Despite sufficient evaluation of the classification, SUE accounted for a substantial proportion of LVO patients. When the LVO patients were applied to the SSS TOAST system, cryptogenic embolism can be independently distinguished from SUE. Therefore, cryptogenic embolism is required in stroke etiology classification for LVO patients receiving EVT, as in the SSS TOAST classification.
The classic TOAST classification reported in 1993 has been the most widely used system for classifying stroke etiology and is based on a thorough etiological workup. Whereas it was designed for secondary prevention, EVT was not considered when it was designed [1]. The occurrence of cryptogenic embolism is substantial among patients with LVO who received EVT [12], but it is not included in the TOAST classification. In addition, if a major cerebral artery is occluded and no cardioembolic source is found, then the case can be erroneously classified as LAA. The SSS TOAST classification reported in 2005 was thought to be more appropriately designed in the current EVT era wherein cryptogenic embolism was also defined [2]. One of the three criteria considers the imaging evidence of the complete recanalization of a previously occluded artery without a cardioembolic source [2]. In addition to the SSS TOAST, the cause could be ascertained more conclusively if a repeat angiographic evaluation is performed between 24 hours and 7 days after EVT. During that period, partially cleared clots could get completely resolved, and some suspicious stenosis would be even more evident. A repeat computed tomographic angiography with the same protocol as the baseline imaging would be useful for patient management in most situations, but digital subtraction angiography could be necessary in certain cases. Additionally, in the SSS TOAST classification, embolic infarctions with 50% or less stenosis can still be classified as LAA [3]. Since the classification is evidence-based, certain cases initially SUE from the classic TOAST can be reclassified as LAA, CE, and other categories to the SSS TOAST [3]. In our study, patients initially classified as SUE were reassigned to the LAA and CE categories.
From the EVT perspective for intracranial LVO treatment, intracranial and extracranial LAA should be distinguished, because underlying stenosis may persist in intracranial LAA, whereas extracranial LAA can achieve full recanalization by mechanical thrombectomy. Artery-to-artery embolism causing intracranial LVO can be migrated from in situ thrombosis of the extracranial LAA whereas in situ thrombosis of the intracranial LAA can cause LVO itself. In terms of procedural factors, despite being treated with EVT, the elevated incidence of partial recanalization in patients with ICAS necessitates distinct approaches in terms of preventing and managing future stroke recurrence following an initial stroke. In addition, reocclusion in ICAS is frequent, and the puncture-to-recanalization (procedure) time is also delayed [13]. Therefore, ECAS and ICAS should be considered separately for EVT strategies. Moreover, since EVT for AIS primarily targets intracranial occlusions, there is a necessity for a distinct classification system to accurately assess the effectiveness of EVT in individuals with acute external carotid artery occlusion [14,15].
In cases where the etiology of stroke remains undetermined despite extensive testing, additional invasive diagnostic procedures such as implantable cardiac monitors or transesophageal echocardiography may be necessary to identify the underlying cause. Patients with cryptogenic etiology require closer monitoring after acute stroke treatment compared to those with a clearly identified cause [16]. This is because paroxysmal atrial fibrillation is often detected in many cryptogenic cases, and in such instances, anticoagulation therapy may be more effective than antiplatelet therapy. It is important to differentiate these patients from those with multiple etiologies (SUE) as they may require different approaches in terms of prevention and treatment strategies.
The existing ASCO (A: atherosclerosis; S: small-vessel disease; C: cardiac pathology; O: other causes) phenotyping system and the MAGIC (MRI-based diagnostic algorithm for AIS subtype classification) system can potentially incorporate the differentiation between ECAS and ICAS [17,18]. However, these classification systems are even more complex and less familiar to many clinicians. To effectively incorporate the differentiation of ECAS and ICAS into stroke classification, it is more practical to modify the existing TOAST and SSS TOAST classifications, as they are widely recognized and utilized in clinical practice. Currently, a significant focus in EVT research is on ICAS. However, it is important to consider the potential need for emergency stenting in cases of extracranial carotid artery stenosis causing acute stroke [6]. To effectively evaluate the outcomes of such treatments, a proper classification system is necessary. Furthermore, a suitable classification system is vital for the future prevention and follow-up of stroke patients with ECAS. Although more accurate and detailed classifications have been proposed, the TOAST and SSS TOAST classifications remain widely utilized due to their simplicity and long-standing presence. However, there is a need to adapt these classifications to current standards [5]. Our study proposes a modified system from the SSS TOAST classification, which offers the advantage of being user-friendly and widely accessible. While our reclassification has its limitations, it will serve as a starting point for further discussions that can lead to a classification system. It will need expert consensus and further large-scale validation studies.
This study has some limitations. It is a retrospective study conducted at a single center, which may limit the generalizability of our findings. Therefore, further investigations involving multiple centers are warranted to obtain more robust and conclusive results. In addition, a validation evaluation lacked in the current study and should also be included in a future large-scale study. However, we are confident in the reliability of this modification, which entails distinguishing between ICAS and ECAS within the LAA category of the SSS TOAST. The classification process for this modification does not present significant challenges, and we believe it can be reliably applied in practical settings.
In conclusion, we propose a novel LVO classification system that divides the LAA category into ECAS and ICAS in the SSS TOAST and adds cryptogenic etiology. Characterized by a pilot study, this modified classification system aims to motivate experts for more refined classification of LVO and EVT patients.
Notes
AUTHOR CONTRIBUTIONS
Conception or design: MK, JSL.
Acquisition, analysis, or interpretation of data: MK, JSL.
Drafting the work or revising: MK, SJL, SYP, JMH, JSL.
Final approval of the manuscript: MK, JSL.
Fig. 1.
Proportions of etiological subgroups in overall acute ischemic stroke population and large vessel occlusion (LVO) population. LAA, large artery atherosclerosis; CE, cardioembolism; SAO, small artery occlusion; SUE, stroke of undetermined etiology; SOE, stroke of other determined etiology; TOAST, Trial of Org 10172 in Acute Stroke Treatment; AIS, acute ischemic stroke; EVT, endovascular treatment; SSS TOAST, Stop Stroke Study TOAST.
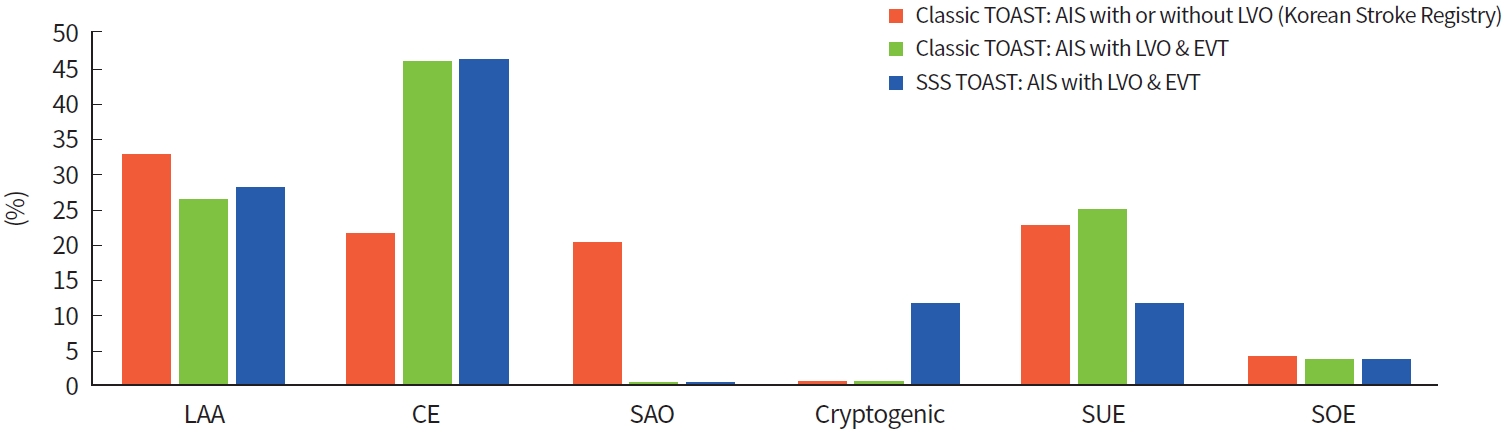
Fig. 2.
Proportions of etiological subgroups by large vessel occlusion (LVO) classification. ECAS, extracranial atherosclerosis; ICAS, intracranial atherosclerosis; CE, cardioembolism; SUE, stroke of undetermined etiology; SOE, stroke of other determined etiology.
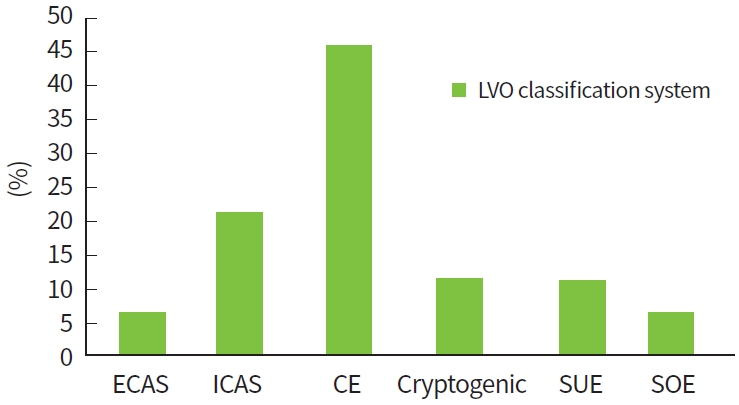
Table 1.
Applying patients with intracranial large vessel occlusion and EVT to a novel classification system
Values are presented as median (interquartile range), number (%), or mean±standard deviation.
EVT, endovascular treatment; ECAS, extracranial atherosclerosis; ICAS, intracranial atherosclerosis; CE, cardioembolism; SUE, stroke of undetermined etiology; SOE, stroke of other determined etiology; NIHSS, National Institutes of Health Stroke Scale.
Table 2.
Comparison of previous stroke and a new LVO etiological classification systems
| TOAST classification [1] | |
| Advantage | A traditional classification in use for a long time based on thorough etiological evaluations |
| Limitation | Designed for secondary prevention and somewhat obsolete |
| LVO can be misclassified unless it is recanalized | |
| No entity for cryptogenic embolism | |
| Extracranial atherosclerosis is an embolic cause from the MT standpoint | |
| All thorough evaluations are not always possible especially for serious patients with LVO and EVT | |
| SSS TOAST classification [2] | |
| Advantage | A renewed classification system based on thorough etiological evaluations |
| Various types of LVO can be more appropriately classified | |
| Entities for cryptogenic embolism are present | |
| Limitation | All thorough evaluations are not always possible especially for serious patients |
| Extracranial atherosclerosis is an embolic cause from the MT standpoint | |
| Baseline angiography is mostly used for determining vascular status | |
| LVO classification | |
| Advantage | Distinguishes ECAS and ICAS |
| Entities for cryptogenic embolism are present | |
| Easy to access because it is a modification of SSS TOAST | |
| Suggestion | Repeat of the angiographic evaluations performed between 24 h and 7 days after EVT |
REFERENCES
1. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41.


2. Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol 2005;58:688–97.


3. Kim BJ, Kim JS. Ischemic stroke subtype classification: an Asian viewpoint. J Stroke 2014;16:8–17.



4. Ding D. Endovascular mechanical thrombectomy for acute ischemic stroke: a new standard of care. J Stroke 2015;17:123–6.




5. Adams HP Jr, Biller J. Classification of subtypes of ischemic stroke: history of the trial of org 10172 in acute stroke treatment classification. Stroke 2015;46:e114–7.

6. Collette SL, Rodgers MP, van Walderveen MA, Compagne KC, Nederkoorn PJ, Hofmeijer J, et al. Management of extracranial carotid artery stenosis during endovascular treatment for acute ischaemic stroke: results from the MR CLEAN Registry. Stroke Vasc Neurol 2023;8:229–37.


7. Lee JS, Lee SJ, Hong JM, Alverne FJ, Lima FO, Nogueira RG. Endovascular treatment of large vessel occlusion strokes due to intracranial atherosclerotic disease. J Stroke 2022;24:3–20.




8. Lee JS, Lee SJ, Yoo JS, Hong JH, Kim CH, Kim YW, et al. Prognosis of acute intracranial atherosclerosis-related occlusion after endovascular treatment. J Stroke 2018;20:394–403.




9. Abdalla RN, Cantrell DR, Shaibani A, Hurley MC, Jahromi BS, Potts MB, et al. Refractory stroke thrombectomy: prevalence, etiology, and adjunctive treatment in a North American cohort. AJNR Am J Neuroradiol 2021;42:1258–63.



10. Yu KH, Bae HJ, Kwon SU, Kang DW, Hong KS, Lee YS, et al. Analysis of 10,811 cases with acute ischemic stroke from Korean Stroke Registry: hospital-based multicenter prospective registration study. J Korean Neurol Assoc 2006;24:535–43.
11. Jeong HY, Jung KH, Mo H, Lee CH, Kim TJ, Park JM, et al. Characteristics and management of stroke in Korea: 2014-2018 data from Korean Stroke Registry. Int J Stroke 2020;15:619–26.



12. Lee JS, Hong JM, Lee KS, Suh HI, Demchuk AM, Hwang YH, et al. Endovascular therapy of cerebral arterial occlusions: intracranial atherosclerosis versus embolism. J Stroke Cerebrovasc Dis 2015;24:2074–80.


13. Rubiera M, Ribo M, Delgado-Mederos R, Santamarina E, Delgado P, Montaner J, et al. Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke 2006;37:2301–5.


14. Lin CH, Saver JL, Ovbiagele B, Tang SC, Lee M, Liebeskind DS. Effects of endovascular therapy for mild stroke due to proximal or M2 occlusions: meta-analysis. J Neurointerv Surg 2023;15:350–4.


15. Gliem M, Lee JI, Barckhan A, Turowski B, Hartung HP, Jander S. Outcome and treatment effects in stroke associated with acute cervical ICA occlusion. PLoS One 2017;12:e0170247.



16. Yaghi S, Bernstein RA, Passman R, Okin PM, Furie KL. Cryptogenic stroke: research and practice. Circ Res 2017;120:527–40.









