Co-occurrence of leukemia, Crohn’s disease, and tuberous sclerosis in one patient
Article information
Abstract
Leukemia and Crohn’s disease are both diseases that can be influenced by genetic factors. Tuberous sclerosis complex (TSC) is a rare autosomal dominant neurogenetic disorder caused by genetic mutation. This report presents a pediatric case of concomitant development of leukemia, Crohn’s disease, and TSC. We aimed to study the pathogenesis of each disease and report this case to provide data for future reports and research on correlation of these three diseases.
INTRODUCTION
Leukemia and Crohn’s disease are influenced by various environmental and immunological factors; however, these diseases can be partially influenced by genetics. For example, leukemia includes cytokine receptor like factor 2 (CRLF2) and paired box 5 (PAX5) alterations, tumor protein p53 (TP53), CREB binding protein (CREBBP), and Ets-related gene (ERG) mutations; Crohn’s disease includes nucleotide-binding oligomerization domain 2 (NOD2), interleukin 23 receptor (IL23R), and TNF superfamily member 15 (TNFSF15) mutation [1,2]. Tuberous sclerosis complex (TSC) is a rare autosomal dominant neurogenetic disorder caused by mutations in either the TSC1 or the TSC2 gene [3]. The relationships between these genes are yet to be elucidated.
Previous reports have described Crohn’s disease and TSC occurring simultaneously in one patient [4,5]. However, the co-occurrence of these two diseases and leukemia has not been reported. The co-occurrence of three uncommon diseases in one patient may not be related. However, if there is a connection, it is possible that genetic factors have played a role.
This case report details the co-occurrence of leukemia, Crohn’s disease, and TSC developing simultaneously in a pediatric patient. We aimed to study the pathogenesis of each disease and report this case to provide data for future reports and research on correlation of these three diseases.
CASE REPORT
A 12-year-old boy presented to the outpatient clinic with nausea, epigastric pain, and poor weight gain for the past 2 months. His birth history was unremarkable. However, he was previously diagnosed with acute B cell lymphoblastic leukemia (B-ALL) 7 years ago and was undergoing routine follow-up with no complication after chemotherapy. The patient was admitted to the hospital for further evaluation.
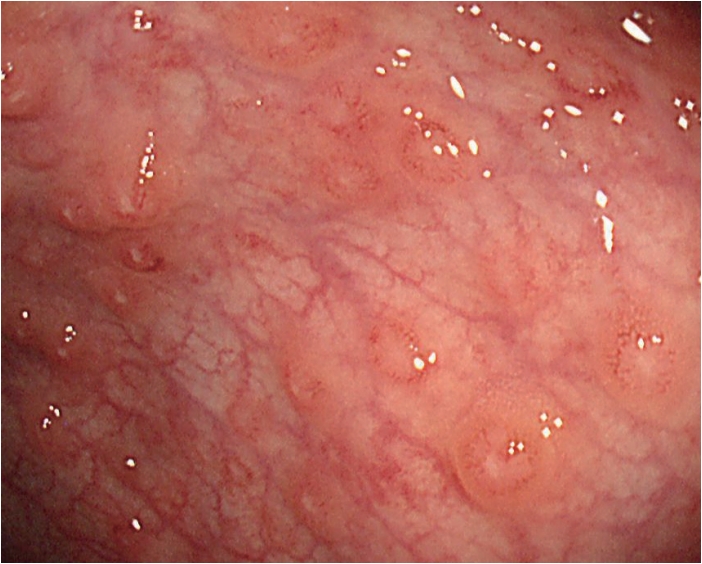
Blood tests (including complete blood count, peripheral blood smear, chemistry profile, and inflammatory markers), esophagogastroduodenoscopy (EGD), fecal calprotectin, and brain magnetic resonance imaging (MRI) were included in the initial evaluation. Since the previous brain MRI performed during the past B-ALL treatment showed chemotherapy-induced toxic leukoencephalopathy, we decided to perform it again for the purpose of follow-up. No abnormal results were noted in the blood tests except hypoalbuminemia at 3.6 g/dL. In the EGD, a single nodular ulcer was observed on the pylorus. In addition, the fecal calprotectin was high at 1,974.0 mg/kg, suggesting inflammatory bowel disease. Therefore, colonoscopy was performed as an additional evaluation. During the colonoscopy, multiple aphthous ulcers were found in the terminal ileum, and from the descending colon to the rectum (Fig. 1). The result of biopsies obtained several days later revealed a non-caseating granuloma. Consequently, the patient was diagnosed with Crohn’s disease, and medical treatment was initiated.
Separately, multiple non-enhancing subependymal nodules were identified in both lateral ventricles, and multifocal lineal non-enhancing high signal intensity lesions were identified in the cortex gray matter and deep white matter of both the frontal lobes and both the insula on brain MRI performed during hospitalization (Fig. 2). The MRI findings indicate the typical presence of tuberous sclerosis. In a belatedly reevaluated physical examination, multiple hypopigmented macules measuring between 0.5 and 2.5 cm in size on the skin of his trunk, arms, and legs (Fig. 3), were identified. Additional neurological examination revealed no abnormalities. Results of TSC1 gene sequencing, which was obtained 1 month later, revealed a heterozygous mutation with DNA change of c.1808dup, leading to a premature stop codon in exon 15. This mutation is not observed in general databases and has been reported in other patients with TSC. Therefore, the mutation was classified based on the American College of Medical Genetics and Genomics (ACMG) guidelines and confirmed as a pathogenic variant [6].
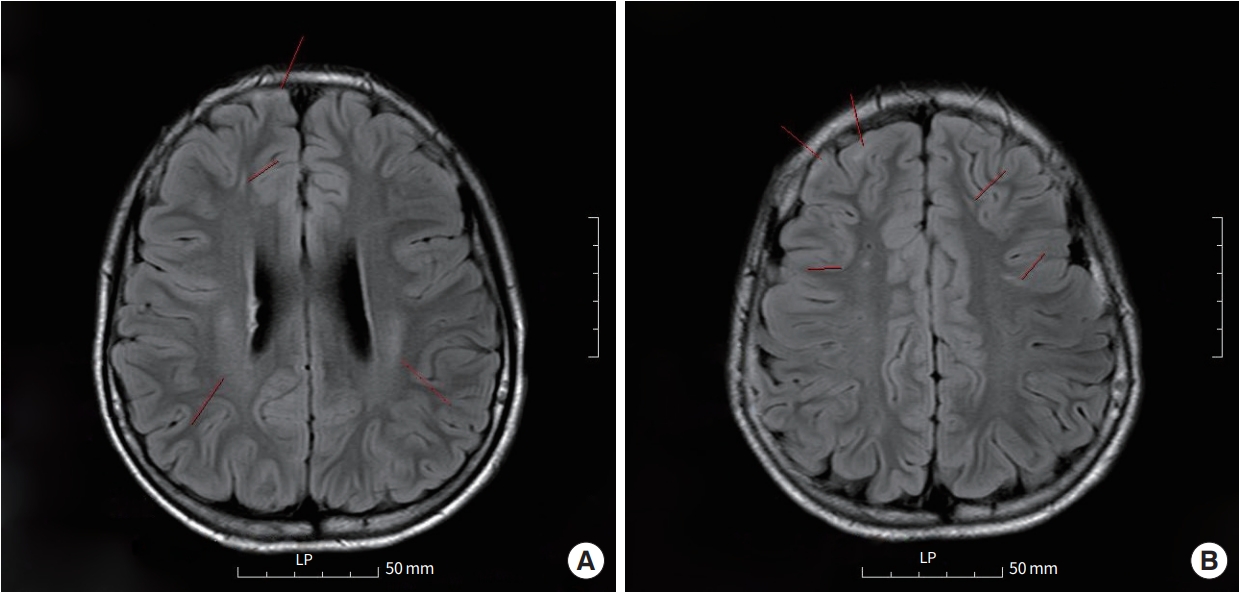
Abnormal findings confirmed on the patient's brain magnetic resonance imaging examination. (A) Multiple non-enhancing subependymal nodules and lineal non-enhancing high signal intensity lesion identified on brain magnetic resonance imaging. The red line indicates the location of the lesion. (B) A photo with the same description of the disease state as (A) but at a different level.
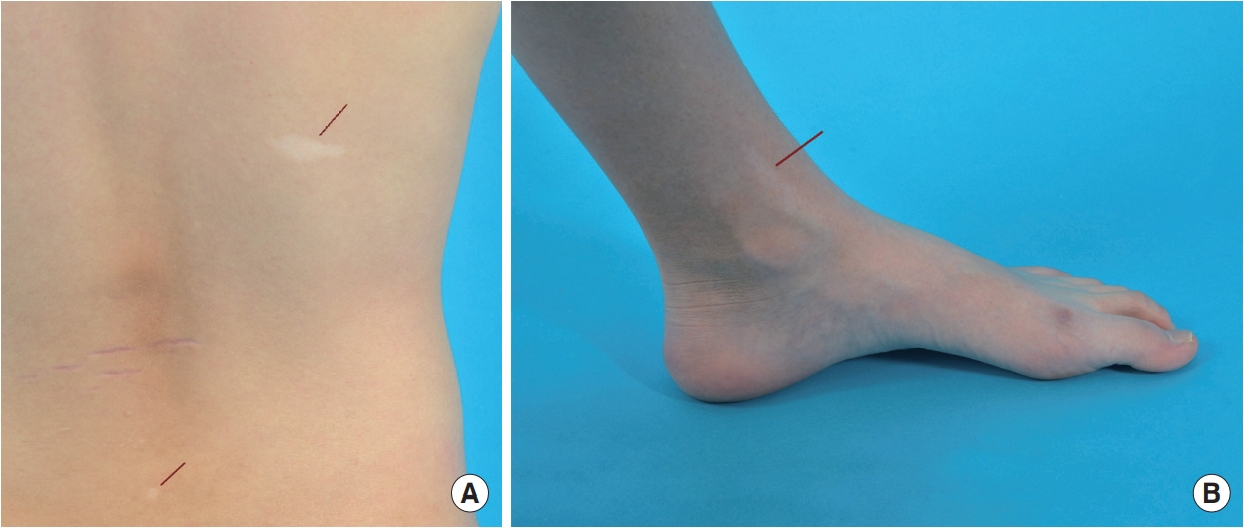
Hypopigmented macules on the skin observed in the patient. The red line indicates the location of the lesion. (A) Hypopigmented macules on the patient's back. (B) Hypopigmented macule in the patient's left ankle.
The medical records of the patient were reviewed retrospectively with the approval of the Clinical Research Ethics Committee of Inha University Hospital, and the requirement for a consent form was waived (IRB file no.: 2023-07-020).
DISCUSSION
Despite differences by region, the approximate prevalence of B-ALL is reported to be 3.4 per 100,000, the prevalence of Crohn’s disease is reported to be 10 to 20 per 100,000, and the prevalence of TSC is reported to be 1 in 6,000 live births. Although the occurrence of each of these diseases is not rare, the co-occurrence of these three diseases in one patient is extremely rare.
The TSC1 gene encodes a protein called hamartin. Due to the loss of function of hamartin, the mammalian target of rapamycin (mTOR) mechanism is activated, leading to tumor formation. The exact relationship between the three diseases is unknown. However, upon reviewing previous literature, we found reports of leukemia co-occurring with tuberous sclerosis [7,8]. These reports commonly hypothesize that hematologic malignancy is also affected by a mechanism caused by dysregulated mTOR pathway, which causes cell cycle progression and tumor formation.
Representative gastrointestinal manifestations of TSC are hamartomatous polyps, papilloma and fibroma. As a related disease, there are no conditions that cause intestinal inflammation similar to Crohn’s disease. The only cases of enteritis occurring as a side effect are those following the use of everolimus as a treatment for TSC [9]. Although there is no study on the direct relationship between TSC and Crohn’s disease, some reports indicate that the phosphatidylinositol 3-kinase (PI3K)/AKT (protein kinase)/mTOR pathway, which is the pathway for the development of TSC, is related to the development of Crohn’s disease [10-13]. They are commonly evaluated to be related to Crohn’s disease and the phosphatase and tensin homolog (PTEN) gene that regulates this pathway.
In conclusion, it cannot be definitively stated that the three diseases are related due to a lack of conclusive evidence. We report this case to provide data for additional mechanistic research on a possible relationship between the three disease due to complex interactions between genes and signaling pathway molecules that have not been fully identified. Understanding the TSC1 gene and mTOR signaling provides an opportunity to recognize a wider range of diseases beyond TSC.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: YK (Yiyoung Kwon).
Acquisition, analysis, or interpretation of data: YJA, DP.
Drafting the work or revising: MJK, YHC, YK (Yiyoung Kwon).
Final approval of the manuscript: YJA, DP, MJK, YHC, YK (YoungseKwon), YK (Yiyoung Kwon).