Central positional nystagmus due to acute medial medullary infarction
Article information
Abstract
A 70-year-old male with risk factors for vascular disease presented with dizziness and instability. The patient showed horizontal geotropic nystagmus when turning his head while lying down, with larger slow phase velocity observed when turning to the left. There were no other neurological deficits. Video head impulse tests and vestibular evoked myogenic potentials were normal. The initial magnetic resonance imaging (MRI) findings were negative; however, the patient experienced subsequent right-side weakness, right tilting, and recurrent episodes of vertigo. A follow-up MRI revealed an acute left medial medullary infarction, which seems to have caused the paroxysmal geotropic central positional nystagmus in this patient.
INTRODUCTION
Central positional nystagmus (CPN) refers to nystagmus triggered by a change in head motion with respect to gravity, as a result of disease affecting central vestibulo-cerebellar pathways [1]. CPN can be divided into two forms. The paroxysmal form is associated with post-rotatory rebound nystagmus due to lesions involving the cerebellar nodulus and uvula, while persistent CPN may be caused by erroneous gravity estimation [2]. While CPN is commonly caused by lesions involving the cerebellum and brainstem, reports of brainstem lesions are less frequent [3]. Here, we report a case of paroxysmal CPN caused by acute medial medullary infarction.
CASE REPORT
A 70-year-old male with a history of diabetes mellitus and hypertension presented to the emergency department with complaints of dizziness, rightward tilt when walking, and subjective right-side weakness. During neurological examination, the patient was alert, oriented, and attentive. There were no cranial nerve damage signs, motor weakness, or cerebellar signs such as limb dysmetria. However, during neuro-otological examination, the patient experienced brief episodes of dizziness and horizontal geotropic nystagmus triggered by head turns in the supine position, with larger slow phase velocity (SPV) observed when turning to the left. The patient was admitted to our hospital for further evaluation. Initial brain magnetic resonance image (MRI) findings did not reveal pathology other than multiple preexisting lacunar ischemic lesions in the bilateral basal ganglia and thalamus. Video nystagmography (VNG) revealed continuous low SPV left horizontal, downbeat nystagmus without fixation (Fig. 1). There was no abnormal gaze-evoked nystagmus nor any asymmetries in smooth pursuit and saccades. Upon positional/positioning maneuvers, turning the patient’s head to the left in the supine position provoked a strong geotropic horizontal (maximal SPV, 40.6°) and counterclockwise (CCW) nystagmus that occurred nearly without a latency and a duration of 22 seconds. A similar left horizontal and CCW nystagmus was also provoked during left Dix-Hallpike head hanging. In contrast, turning the head to the right in the supine position provoked only a subtle geotropic horizontal nystagmus, whereas right Dix-Hallpike head hanging provoked a strong right horizontal (maximal SPV, 23.7°), downbeat, clockwise (CW) nystagmus with no latency and duration of 12 seconds. Left Dix-Hallpike sitting resulted in right horizontal downbeat nystagmus, and right Dix-Hallpike sitting provoked downbeat nystagmus. Neither video head impulse tests nor cervical and ocular vestibular evoked myogenic potentials showed any abnormalities. Considering the differential diagnosis of left horizontal canal canalolithiasis, Barbecue roll maneuver was performed two times, and the patient’s nystagmus improved. As the patient’s dizziness improved the next day without any focal neurological deficits and brain imaging findings did not reveal central pathology, the patient was discharged and prescribed antiplatelet medication, and a short clinical follow-up was planned. The patient returned to the clinic 7 days later, complaining of right-side weakness, right tilting, and recurrent episodes of vertigo. He was readmitted to the hospital, and a follow-up brain MRI scan revealed a new diffusion restriction in the anteromedial aspect of upper medulla (Fig. 2). Follow-up VNG during positional maneuvers revealed continuous left horizontal geotropic nystagmus upon left head turn in supine position (latency, 8 seconds), continuous downbeat nystagmus upon left Dix-Hallpike sitting (latency, 6 seconds), continuous upbeat and CW torsional nystagmus upon right Dix-Hallpike hanging (latency, 6 seconds), and continuous left horizontal downbeat nystagmus upon right Dix-Hallpike sitting (latency, 8 seconds). After acute stroke treatment, the patient was discharged, and his symptoms of dizziness and imbalance improved after 1 month.
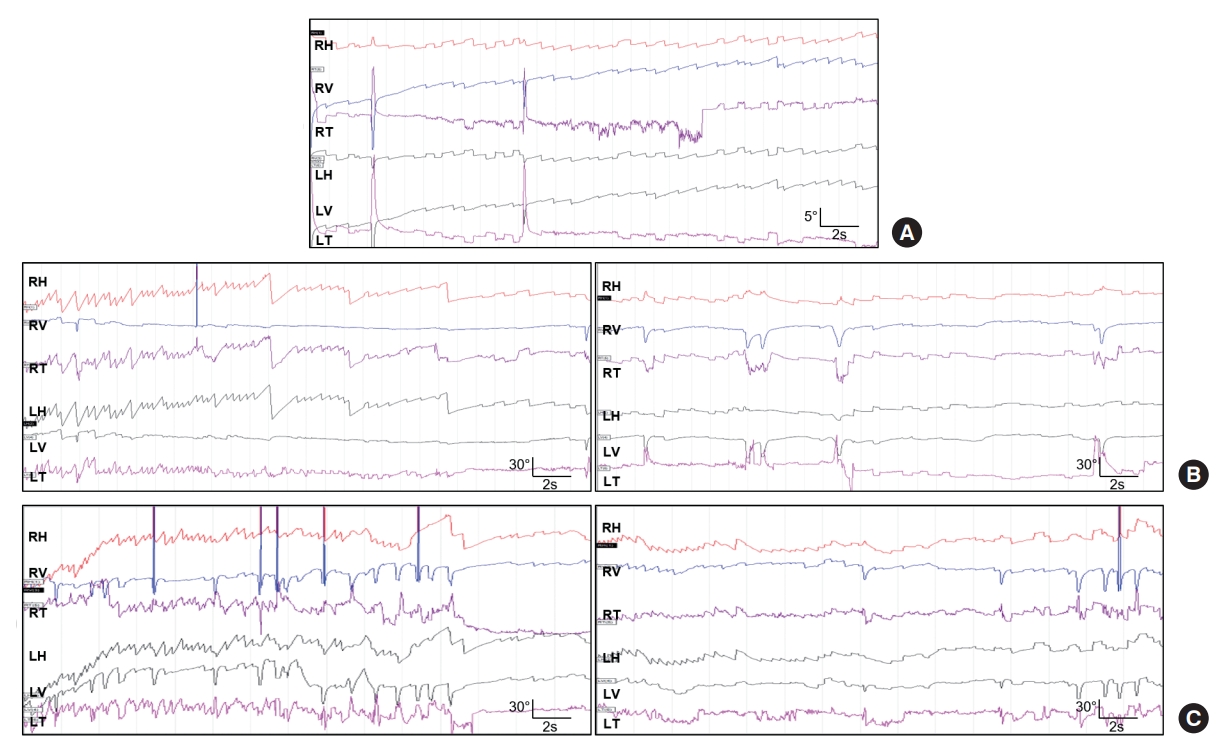
Video nystagmography of the current patient shows (A) weak spontaneous nystagmus, (B, left panel) strong paroxysmal geotropic nystagmus upon left head turn, while (B, right panel) right head turning evoked only a subtle geotropic horizontal nystagmus. Left Dix-Hallpike maneuver (C, left panel) and right Dix-Hallpike maneuver (C, right panel) also elicit paroxysmal positional nystagmus. RH, right horizontal; RV, right vertical; RT, right torsional; LH, left horizontal; LV, left vertical; LT, left torsional.
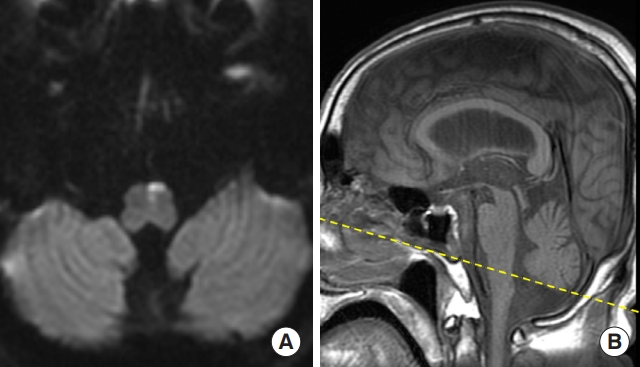
Brain magnetic resonance imaging shows acute infarction involving the medial aspect of the upper medulla. (A) Axial image shows diffusion restriction in the anteriomedial aspect of upper medulla. (B) Sagittal T1 imaging shows the level of the diffusion restriction marked by yellow dotted line.
Written informed consent by the patient was waived according to Ajou University Hospital Institutional Review Board guidelines due to the retrospective nature of our study.
DISCUSSION
The authors report a case of paroxysmal CPN in which diffusion weighted MRI scan was initially negative. A follow-up MRI revealed acute medial medullary infarction. Initially, the patient showed ipsilesional spontaneous nystagmus and ipsilesional paroxysmal geotropic nystagmus. After a few days, the nystagmus changed to a persistent form of CPN.
The mechanism of paroxysmal geotropic nystagmus has not been well elucidated. For paroxysmal apogeotropic nystagmus, primary nodulus and uvula dysfunction may result in disinhibition of the semicircular canal-driven signals reaching the brainstem, causing a prominent post-acceleratory rebound phenomenon. It will also initiate a deficient rotational feedback mechanism that cannot compensate in a real-time fashion for a large difference between an erroneous estimation of gravity direction and its actual direction [1,4]. For persistent geotropic nystagmus, a case series has shown that tonsillar dysfunction may result in compensatory rotational feedback due to erroneous estimation of the direction of gravity, which may generate constant horizontal geotropic positional nystagmus [5]. Horizontal linear vestibulo-ocular reflex disorder may also result in horizontal geotropic positional nystagmus [6].
As dysfunction of central vestibulocerebellar pathways are responsible for the occurrence of CPN, a medial medullary infarct has seldom been reported as a cause of CPN. We believe that involvement of the nucleus prepositus hypoglossi (NPH) may be responsible for the geotropic CPN seen in the current patient. One case of apogeotropic positional nystagmus has been reported with an old infarction of the brainstem around the NPH [7]. In this case, the authors characterized the apogeotropic positional nystagmus as prolonged post-rotatory nystagmus due to the enhanced velocity storage mechanism. The NPH acts as a horizontal neural integrator and participates in the vestibulo-cerebello-oculomotor unit [8]. Currently, lesions of the NPH are known to cause ipsilesional-beating spontaneous nystagmus, horizontal gaze-evoked nystagmus, impaired ipsilesional pursuit, central patterns of head-shaking nystagmus, contralateral eye deviation, and decreased vestibulo-ocular reflex gain during contralesionally directed head impulses [9]. The current study shows that ipsilateral dominant geotropic CPN may also occur with NPH lesions.
Due to the initial negative MRI, the symptoms were initially considered a result of a left horizontal canal canalolithiasis. Clinically, it is important to differentiate central from peripheral positional nystagmus. Some clinical clues for this patient included: (1) the direction of nystagmus was not in plane of the stimulated canal; (2) absence of latency following positioning maneuver; and (3) the patient’s complaint of transient subjective right-side weakness [3]. While it is also important to confirm the disappearance of positional nystagmus after repositioning therapy, we are not sure why there was initial improvement of nystagmus after Barbecue roll maneuver in this patient. It may have been due to fatiguability, which is known to occur in some cases of CPN [3]. Practically, clinicians should watch out for any characteristics that raises suspicion of CPN [10].
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: SJL.
Acquisition, analysis, or interpretation of data: SJL, YSK, JSL, JMH.
Drafting the work or revising: SJL.
Final approval of the manuscript: SJL, YSK, JSL, JMH.
