A case of acute invasive fungal sinusitis in an immunocompetent patient on glatiramer acetate therapy
Article information
Abstract
Acute invasive fungal sinusitis (AIFS) is a rapidly progressing, life-threatening infection. The advent of immunomodulatory therapies has expanded the population susceptible to AIFS. In this case report, we describe a patient who defies the conventional profile of AIFS. This 70-year-old woman is immunocompetent and non-diabetic, with a history of multiple sclerosis (MS) and ongoing treatment with glatiramer acetate (GA), immunomodulator. She came to the emergency room due to acute vision changes, and images revealed an enhancing mass in the left pterygopalatine fossa. The diagnosis of AIFS was confirmed by biopsy in the operating room. Subsequently, anti-fungal therapies were initiated with a follow-up operative debridement. Follow-up magnetic resonance imaging (10 months since treatment) showed no progression of AIFS. GA was discontinued since the diagnosis, and MS has remained stable. Recognizing this unique group of atrisk patients is crucial, as early detection and treatment play a pivotal role in preventing significant morbidity and mortality.
INTRODUCTION
Acute invasive fungal sinusitis (AIFS) is a life-threatening, rapidly progressing, angioinvasive infection occurring almost exclusively in immunocompromised patients [1]. The presentation can be quite variable, with some series reporting orbital findings in up to 50% of patients [2]. Poorly controlled diabetes, chronic immunosuppression, and hematologic malignancies account for 90% of the causes of AIFS, while autoimmune disease is estimated to cause about 1.2% of cases [3]. The latter represents a unique population in which a compromised immune system may not be required to develop AIFS.
Very few studies discuss such patients without the typical risk factors for AIFS. Kasapoglu et al. [4] reported on 26 patients with AIFS, describing one patient with rheumatoid arthritis and another with systemic lupus erythematosus (SLE). Details were not available regarding the medications they were taking. Rangel-Guerra et al. [5] reported on 36 patients with AIFS, describing one patient with SLE but again not detailing medication history. Such patients present a challenge to our central dogma of AIFS, in which immunocompromise or poorly controlled diabetes mellitus is required for clinical suspicion. Perhaps there is an undiscovered link between immune-modulating therapies that predispose patients with autoimmune conditions to this opportunistic infection.
Glatiramer acetate (GA) is considered a non-biologic, non-interferon polymer widely used as a disease-modifying agent in treating relapsing forms of multiple sclerosis (MS) [6]. The exact immune reaction of GA is largely unknown, but it is believed to be involved in multiple immunomodulatory processes, including generating suppressor cells, inducing tolerance, expanding regulatory T-cell populations, and altering antigen-presenting cells [6]. This report aims to present the case of an immunocompetent patient diagnosed with AIFS and call attention to a new subset of patients at risk for this disease process.
CASE REPORT
A 70-year-old female with a history of MS, hypertension, and hyperlipidemia presented to the University of Alabama at Birmingham Hospital to investigate a new orbital mass. The patient reported a 3-day history of acute visual disturbance that rapidly progressed to complete loss of light perception on the day of presentation. Additionally, she described mild left-sided nasal congestion and facial pressure. A physical exam demonstrated a left relative afferent pupillary defect with no other cranial neuropathies. Fiberoptic nasal endoscopy revealed healthy, pink, and sensate nasal mucosa without evidence of necrosis or ischemia. Computed tomography (CT) scan (Fig. 1) and magnetic resonance imaging (MRI) (Fig. 2A) showed an irregularly heterogeneously enhancing mass filling the left pterygopalatine fossa (PPF) and inferior orbital fissure (IOF) with extension and erosion into the maxillary sinus, left sphenoid sinus, left superior orbital fissure, and orbital apex (Fig. 2B).
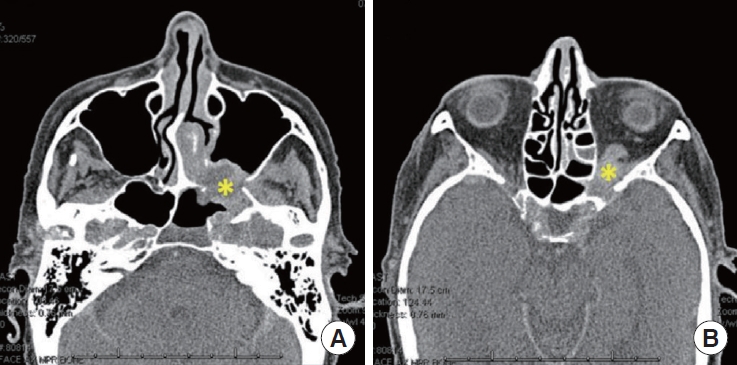
Computed tomography sinus with contrast. (A) Axial cut showing the heterogeneously enhancing mass (yellow asterisk) origination in the left sphenopalatine area with erosion of the posterior maxillary wall. (B) The mass (yellow asterisk) extends into the orbit with the involvement of the orbital apex.
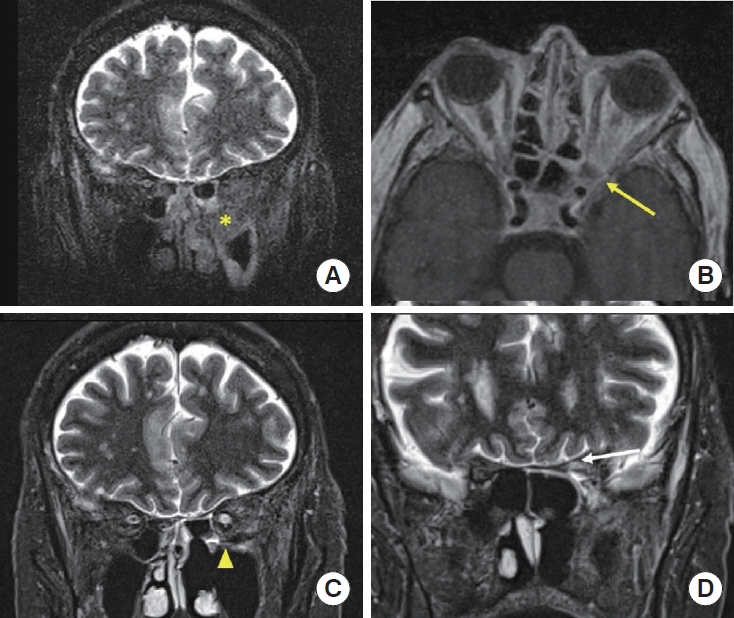
Brain magnetic resonance imaging with pre- and post-treatment. (A) Pre-treatment (coronal): Short tau inversion recovery (STIR) signal showed low signal intensity mass located in the left pterygopalatine fossa (yellow asterisk) with bony destruction. (B) Pre-treatment (axial): Spread to the adjacent orbital apex (yellow arrow). (C) Post-treatment (coronal): STIR signal demonstrated an interval near complete resolution of the soft tissue mass of the left pterygopalatine fossa (yellow arrowhead) after 10 months of treatment. (D) Post-treatment (axial): There is a mild T2 hypersignal intensity and volume loss involving the left optic nerve at the location of the left orbital apex and in the prechiasmatic region (white arrow) in favor of sequela of prior optic nerve infarction.
The patient was taken to the operating room and had left transpterygoid approach for a biopsy of the mass on hospital day 1. A polypoid mass enchasing friable and necrotic tissue (Fig. 3) was identified in the left posterior maxillary wall extending into the sphenopalatine area and IOF. Frozen pathology was concerning for AIFS, confirmed on final pathology, showing invasive fungal organisms with focal hyphal angioinvasion (Fig. 4). Tissue culture was positive for Aspergillus fumigatus species. Infectious disease (ID) was consulted, and the patient was initiated on amphotericin nasal irrigations, amphotericin B lipid complex (abelcet) 5 mg/kg intravenous (IV) daily, and posaconazole 30 mg daily. The oculoplastic surgeon recommended against orbital exenteration based on the extent of orbital involvement and disease extending to the left cavernous sinus. The patient underwent operative debridement 7 days after the transpterygoid approach without evidence of disease progression, again yellowish necrotic mass in the PPF and IOF regions. She was discharged on hospital day 10 on oral posaconazole and amphotericin nasal irrigations without IV amphotericin. Clinical exam and brain MRI 10 months after the initial presentation showed no disease progression or skull base invasion (Fig. 2C). Therefore, per ID recommendation, her oral posconazole and amphotericin nasal irrigation were discontinued afterward. The follow-up MRI still demonstrated the findings of prior optic nerve infarction from AIFS (Fig. 2D); the patient remained blind in her left eye. GA has been discontinued since the diagnosis of AIFS without any consequence on her MS.
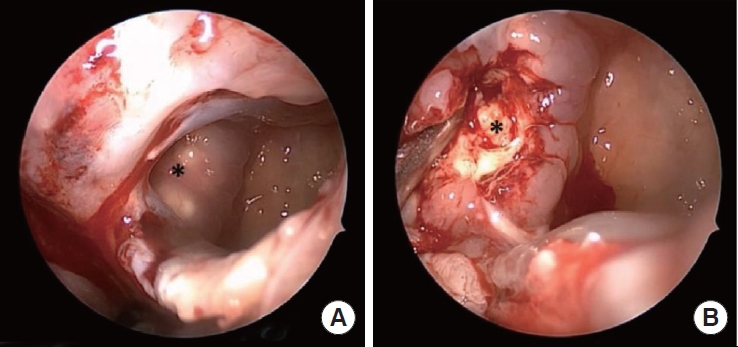
Intraoperative findings. (A) A polypoid mass (asterisk) was identified in the left posterior maxillary wall after opening the maxillary sinus. (B) The mass was filled with friable and necrotic tissue (asterisk) extending into the pterygopalatine fossa.

Histopathologic findings. (A) Mid-power (100×) sinonasal mucosa with prominent mixed inflammation and an area of necrosis and invasive hyphal elements (arrows). (B) Higher power view (200×) of the same area with hyphal elements visible on routine H&E stain (arrows) accompanied by necrosis. (C, D) Other areas of invasive hyphal elements (arrows) with a corresponding high-power view are depicted in images (C) and (D), respectively (×200). (E) Detached fragments of hyphae were also seen, so-called “fungal ball,” noting the lack of pleomorphic features, the overall narrowness of the hyphae, and the “acute angle” branching pattern, all of which suggest Aspergillus spp. organisms (×200). (F) The invasive nature of the fungal microorganisms was highlighted by a Grocott–Gömöri's methenamine silver (GMS) histochemical stain (arrows) with foci of angioinvasion (right upper inset) (×200).
DISCUSSION
AIFS is a life-threatening and rare infection that affects almost exclusively immunocompromised individuals [1]. The most extensive retrospective study done by Turner et al. [3] found an average overall survival rate of 46.1%. AIFS affecting immunocompetent individuals is only reported in a few case reports, most of which are reported from India. One case report by Rangel-Guerra et al. [5] described two immunocompetent patients affected by AIFS, both of whom had indolent courses with symptoms for > 6 months prior to diagnosis. Another report by La Mantia et al. [6] describes a patient who developed invasive mucormycosis 2 weeks after endoscopic sinus surgery for allergic fungal sinusitis.
To our knowledge, this is the first report of AIFS presenting as an orbital mass in an otherwise immunocompetent patient on immunomodulatory therapy for MS. The rarity of this case is even more impressive because symptom onset was acute, which did not follow a prolonged course of symptoms, such as other case reports in the literature, and radiographic presentation was a mass in the PPF and IOF. However, this patient did present with one of the most common symptoms of AIFS, visual change. Then the question is, “Could medications aimed at modifying immune processes to target autoimmune diseases increase the chance of serious opportunistic infections?” Although GA is believed to play a role in modifying T regulatory cells and Th17 cells, natural killer cells, CD4+, and CD8+ T lymphocytes do not appear to be affected in patients on long-term GA therapy. In clinical trials, it has not been shown to increase the risk of any specific opportunistic infections [6]. Nonetheless, as pharmacologic advances are made in the effort to provide long-term symptom relief in autoimmune diseases, the importance of maintaining a high clinical suspicion for AIFS cannot be understated, even in the absence of true immunodeficiency. The more we learn about this disease, the more apparent it is that there is no typical presentation, physical exam, or radiographic finding. Several retrospective studies have shown a high percentage of AIFS patients presenting with normal or near-normal CT scans of the sinuses [7-9].
Here, we present a case describing AIFS in an immunocompetent patient on GA therapy. It is crucial to keep opportunistic infections in mind as part of the differential, even without a true immunodeficiency, as early identification and treatment are critical in avoiding morbidity and mortality.
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: DYC.
Acquisition, analysis, or interpretation of data: MH, JB, BTB.
Drafting the work or revising: MH, JB, BTB, JG, BAW, DYC.
Final approval of the manuscript: BAW, DYC.
Acknowledgements
The abstract of this case report was presented as a poster at the annual meeting of the American Academy of Otolaryngology Head & Neck Surgery 2020.
This work was supported by National Institutes of Health (NIH)/National Institutes of Allergy and Infectious Disease (K08AI146220, 1R21AI168894-01), and Cystic Fibrosis Foundation K08 Boost Award (CHO20A0-KB) to Do-Yeon Cho.
