INTRODUCTION
Superficial siderosis is a pathological condition caused by the deposition of hemosiderin (an iron-storage complex produced by breakdown of red blood cells) in the subpial layer of the central nervous system (CNS), and is diagnosed based on radiological evaluation. From the first description of the disease by Dr. Hamill in 1908, up until 2006, only 270 cases of superficial siderosis were reported [1]. With the widespread use of magnetic resonance imaging (MRI), increasing numbers of cases are being reported. Common causes of superficial siderosis include brain tumors, head and neck trauma, and arteriovenous malformations. However, in 35% of superficial siderosis cases, an occult source cannot be identified.
Herein, we describe two patients diagnosed with superficial siderosis caused by spinal lesions, which is an unusual source of chronic bleeding in superficial siderosis. Both patients were diagnosed using spine MRI and computed tomography (CT) myelography,
CASE REPORTS
Case 1
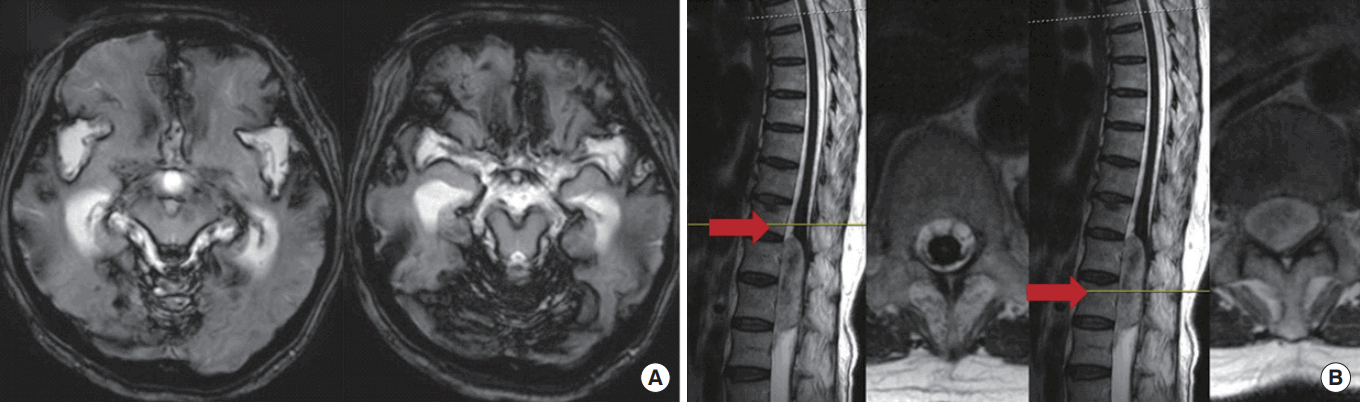
A 63-year-old, previously healthy woman experienced disequilibrium while walking to the grocery store in early 2007. A few months later, she experienced decreased hearing acuity in both ears, which was more prominent in the left ear. A few days later, she complained of mild dysarthria. Thereafter, her symptoms progressed slowly. In 2011, she visited a nearby hospital for evaluation and was diagnosed with idiopathic superficial siderosis and discharged without further management. In 2015, her symptoms worsened, leaving her unable to walk by herself due to severe disequilibrium. The patient visited our outpatient neurological clinic and was admitted for an etiologic evaluation of her superficial siderosis. A review of the patient’s gradient recalled echo (GRE) images showed linear hypointensity along the cerebellar folia and superior vermis, with atrophic changes of the brain stem and cerebellum. An additional spine MRI revealed a collection of epidural fluid in the lower cervical to upper thoracic levels, probably associated with a dural defect. On dynamic CT myelography, epidural contrast leakage was seen in the anterior epidural space of spinal levels C7–T5 (Fig. 1). The patient was treated with an epidural blood patch. One month later, the patient was able to walk without any assistance for 30 minutes and remained stable neurologically.
Case 2
A 60-year-old, previously healthy woman experienced disequilibrium while climbing a mountain in late 2013. In 2014, she complained of frequent falling, the inability to walk for more than 30 minutes, and bradykinesia. A few months later, insidious dysarthria and bilateral hearing difficulties developed and thereafter her symptoms slowly got worse. Eventually, she was unable to sit-up by herself and developed frequent urinary urgency and incontinency. In February 2015, she began to have difficulties in swallowing solid food materials. The patient visited our outpatient neurological clinic and was admitted for evaluation. The patient was alert but had decreased cognitive function (Mini–Mental State Examination, 21/30). She had pathologic limitations in upward gaze and decreased motor power, which was prominent in the right lower extremity (grade I–III), but also present in the other three extremities (grade IV+). She also had bilateral limb ataxia with ataxic gait. A review of the patient’s GRE images showed linear hypointensity along the cerebellar folia, superior vermis, and the cisternal segment of the cranial nerves. Imaging also showed atrophic changes of the brain stem and cerebellum, accompanied by ventriculomegaly. An additional spinal MRI revealed a 6.3-cm-sized intradural mass at the T12 and L1 level, with diffuse superficial siderosis (Fig. 2). The patient was transferred to the neurosurgery department for removal of the tumor. The mass was removed without leaving any residual tumor. The pathology report confirmed that the mass was a myxopapillary ependymoma. Six months following tumor removal, the patient was able to walk 3 to 4 steps without any assistance. The patient is currently undergoing rehabilitation.
DISCUSSION
The clinical features of superficial siderosis are characterized by a classic triad of symptoms consisting of sensorineural hearing loss, cerebellar ataxia, and myelopathy. The features are caused by the preferential involvement of the posterior fossa. This may be partially explained by the presence of Bergmann glia in the cerebellum, that display increased ferritin synthesis [2]. Five steps are required to cause CNS tissue to become siderotic: (1) chronic or intermittent extravasation of blood into the subarachnoid space and subsequent dissemination by the cerebrospinal fluid (CSF), (2) hemolysis, (3) entry of heme into exposed tissue, (4) conversion of heme to free iron, ferritin, and hemosiderin, and (5) damage to nerve tissues [3].
Gradient-echo T2-weighted MRI is the modality of choice for the diagnosis of superficial siderosis, and if the disease is present, it will show a rim of hypointensity around the cerebellum, brainstem, spinal cord, and cerebral convexity. CSF analysis may show xanthochromia, increased protein level, and elevated white blood cell count. However, in many cases, CSF analysis shows normal findings because of the intermittent nature of the bleeding. Recognition of the clinical characteristics of superficial siderosis and the widespread use of MRI will provide an improved chance of early diagnosis and may even allow the discovery of asymptomatic cases. Based on recent improvements in diagnosis, the underlying etiologies of reported cases are as follows: idiopathic (35%), CNS tumor (15%), head/back trauma (13%), arteriovenous malformation (9%), and others (28%) [1]. For the diagnosis of superficial siderosis, CT myelography and whole spine MRI has been proposed for usage in patients without an intracranial source of bleeding [4,5].
Superficial siderosis is a progressive, neurological condition that must be recognized early in order to prevent further progression of neurologic symptoms. Up-to-date, early identification of the source of subarachnoid hemorrhage and ablation, is the only effective way to prevent further neurologic deterioration. The cases presented here emphasize the importance of work ups for the presence of spinal cord lesions in patients with presumed idiopathic superficial siderosis. Further studies are warranted to allow the development of methods to restore the neuronal damage caused by hemosiderin deposition [1]. Case studies have reported beneficial effects of desferrioxamine (an iron chelator), trientine (a copper and iron chelator), and tin protoporphyrin (a heme oxygenase inhibitor) on reversing the neuro toxic effects of hemosiderin deposition.
In conclusion, superficial siderosis is a rare, slowly progressive condition, with potentially severe clinical sequelae. Clinical manifestations vary widely and presentation may mimic a variety of neurological and otorhinolaryngological diseases. Therefore, clinicians should be aware of the disease and include superficial siderosis in the differential diagnosis of cerebellar-pyramidal syndromes. Optimal management remains to be determined, though an early diagnosis with identification and treatment of the bleeding source appears to halt disease progression; thus, improving the prognosis of the patient. Etiologic evaluation, including spine imaging, may be crucial for the early diagnosis and management of the reversible causes of superficial siderosis.