Olaratumab and doxorubicin for the treatment of metastatic soft tissue sarcoma: a retrospective case series
Article information
Abstract
Purpose
Soft tissue sarcomas (STS) are a rare and heterogeneous tumor group with limited treatment options. This study aimed to evaluate the anti-tumor efficacy of olaratumab and doxorubicin in patients with advanced STS in front-line and salvage setting.
Methods
Patients with STS who received olaratumab and doxorubicin between October 2017 and August 2018 were retrospectively reviewed. Response rate, progression-free survival (PFS), and overall survival (OS) were analyzed according to histologic subtype, Eastern Cooperative OncologyGroup performance status, and number of prior chemotherapy regimens.
Results
A total of 26 patients were included in the analysis. The common histologic subtypes included undifferentiated/unclassified sarcoma (n=8), leiomyosarcoma (n=7), liposarcoma (n=3), and solitary fibrous tumor (n=2). Of 26 patients, 10 patients (38.4%) received more than two chemotherapy regimens before olaratumab and doxorubicin. At the time of analysis, 152 cycles of olaratumab had been administered (median, five cycles), and there was no treatment-related mortality. The disease control rate was 61.5% (n=16), and the overall response rate was 15.3% (partial response, n=4; complete response, n=0). Partial responses were achieved in one patient with solitary fibrous tumor, one with dedifferentiated liposarcoma, one with leiomyosarcoma, and one with pleomorphic rhabdomyosarcoma, all in salvage setting. With a median follow-up duration of 11.1 months (95% confidence interval [CI], 11.1 to 13.1 months), the median PFS was 4.1 months (95% CI, 2.1 to 6.1 months), and the median OS was not reached.
Conclusion
Olaratumab and doxorubicin demonstrated acceptable anti-tumor activity in Asian patients with sarcoma.
INTRODUCTION
Soft tissue sarcomas (STS) account for 6% of childhood cancers and 1% of all adult malignancies [1,2]. Among more than 100 subtypes of STS, the most common histologic subtypes are undifferentiated unclassified sarcoma, liposarcoma, leiomyosarcoma, synovial sarcoma and malignant peripheral nerve sheath tumors [3]. Surgical resection with appropriately negative margins is the standard primary treatment for resectable STS, and patients with extremity STS are commonly treated with limb preservation surgery in combination with adjuvant radiotherapy [4]. Chemotherapy with single agents (e.g., dacarbazine, doxorubicin, epirubicin, ifosfamide, pazopanib, trabectedin, or eribulin) or anthracycline-based combination regimens (doxorubicin or epirubicin with ifosfamide and/or dacarbazine) has been widely used for patients with advanced, unresectable, or metastatic disease [5-8].
Olaratumab (Lartruvo, Eli Lilly and Company, Indianapolis, IN, USA) is a fully human immunoglobulin G1 monoclonal antibody that selectively binds the external domain of human platelet-derived growth factor receptor-α (PDGFR) with high affinity and blocks ligand binding [2]. In October 2016, the U.S. Food and Drug Administration granted accelerated approval to olaratumab in combination with doxorubicin (olaratumab+doxorubicin) for the treatment of adult patients with STS with a histologic subtype for which an anthracycline-containing regimen is appropriate and not amenable to curative treatment with radiotherapy or surgery [9].
However, studies on the efficacy and tolerability of olaratumab and doxorubicin in Asian patients with sarcoma are limited. In addition, clinical outcome and tolerability of the regimen in heavily pre-treated patients are scarcely reported. Hence, the efficacy and tolerability of olaratumab and doxorubicin in Asian patients is yet to be established and should be evaluated. Therefore, this retrospective study aimed to evaluate the anti-tumor efficacy of olaratumab and doxorubicin in STS patients in Asia. Particularly, we focused on evaluating the tolerability and anti-tumor efficacy in heavily pre-treated refractory sarcoma patients.
METHODS
Study design
We retrospectively reviewed the medical records of patients with advanced STS who were treated with olaratumab and doxorubicin between October 2017 and August 2018 at Samsung Medical Center (SMC), Korea. Inclusion criteria were as follows: (1) histologically confirmed diagnosis of advanced unresectable or metastatic STS not amenable to curative treatment with surgery or radiotherapy and (2) availability of complete clinical information including patient demographics, primary tumor site, stage, and treatment record. The following clinicopathologic variables were collected: age, sex, histologic type, extent of metastasis, Eastern Cooperative Oncology Group (ECOG) performance status, and treatment history. The study was reviewed and approved by the Institutional Review Board of SMC (IRB No. 2018-08-107), and informed consent was waived.
Treatment
Patients receive olaratumab (15 mg/kg) intravenously on days 1 and 8 plus doxorubicin (75 mg/m²) on day 1 of each 21-day cycle. After eight cycles, patients were allowed to receive olaratumab monotherapy until disease progression provided that they have no disease progression or unacceptable toxicities. During cycles 5 to 8, dexrazoxane was allowed on day 1 of each cycle to reduce the potential for doxorubicin-related cardiotoxicity. Cardiac echography was done for monitoring cardiac function during treatment. Treatment was continued until disease progression, unacceptable toxicity, or patient refusal. Tumor response was evaluated every 6 or 9 weeks using computed tomography scans or magnetic resonance imaging. Responses were assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
Standard descriptive and analytical methods were used to describe the patient population and their baseline characteristics. Overall survival (OS) was defined as the time from the initiation of olaratumab and doxorubicin treatment to the date of death or last follow-up. Progression-free survival (PFS) was defined as the time from initiation of olaratumab and doxorubicin treatment to the date of documented disease progression or death from any cause. Kaplan-Meier curves were used to analyze time-to-event variables, and 95% confidence intervals (CIs) were computed for time-toevent medians. Survival comparisons were performed using univariate log-rank tests. All statistical analyses were performed using R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria), and two-tailed P-values of less than 0.05 were considered statistically significant.
RESULTS
Patient characteristics
Between October 2017 and August 2018, 26 STS patients received olaratumab and doxorubicin. Baseline patient characteristics at the start of olaratumab and doxorubicin treatment are summarized in Table 1. The median age was 53 years (range, 25 to 73 years), and 57.6% of patients were male. Most patients (92.3%) had a good ECOG performance status (0–1). The distribution of histologic subtypes was as follows: eight patients had undifferentiated/unclassified sarcoma; seven, leiomyosarcoma; three, liposarcoma; three, pleomorphic sarcoma; two, solitary fibrous tumor. French Fédération Nationale des Centres de Lutte Contre le Cancer grades were available for eight patients (30.6%); six of these patients (23.0%) had grade 3 sarcoma. The primary tumor site was the trunk or retroperitoneum in 19 patients (73.0%) and the extremities in five patients (19.2%). The most common metastasis sites were the lung (n = 14, 53.8%), liver (n= 7, 26.9%), and bone (n= 4, 15.3%).
A total of 13 patients (50%) of patients previously had at least one chemotherapy regimen before olaratumab and doxorubicin treatment, while the other 13 patients were chemo-naïve. Ten patients (38.4%) were heavily pre-treated with two or more lines of chemotherapy.
A total of nine (34.6%), eight (30.7%), seven (26.9%), and two patients (7.6%) had received prior pazopanib chemotherapy; docetaxel and gemcitabine chemotherapy; etoposide, ifosfamide, and cisplatin chemotherapy, and pembrolizumab chemotherapy, respectively.
A total of 152 cycles of olaratumab and doxorubicin therapy were administered, with a median of five cycles per patient (range, 1 to 16 cycles). At the time of analysis, three patients were still receiving olaratumab and doxorubicin treatment, and the remaining 23 patients had stopped treatment because of progression (n= 21), patient refusal (n= 1), or other reasons (n= 1).
Tumor responses
Of the 26 patients, 25 were evaluable for tumor response except for one patient follow-up loss. No patient achieved a complete response, but four patients achieved partial responses, yielding a 15.3% (95% CI, 1.46% to 29.13%) overall response rate. Partial responses were achieved in one patient with solitary fibrous tumor, one with dedifferentiated liposarcoma, one with leiomyosarcoma, and one with pleomorphic rhabdomyosarcoma. Of the four patients with partial responses, one maintained treatment response for more than 13 cycles at the time of this writing. Twelve patients (46.1%) achieved stable disease, yielding a disease control rate of 61.5% (95% CI, 42.79% to 80.20%).
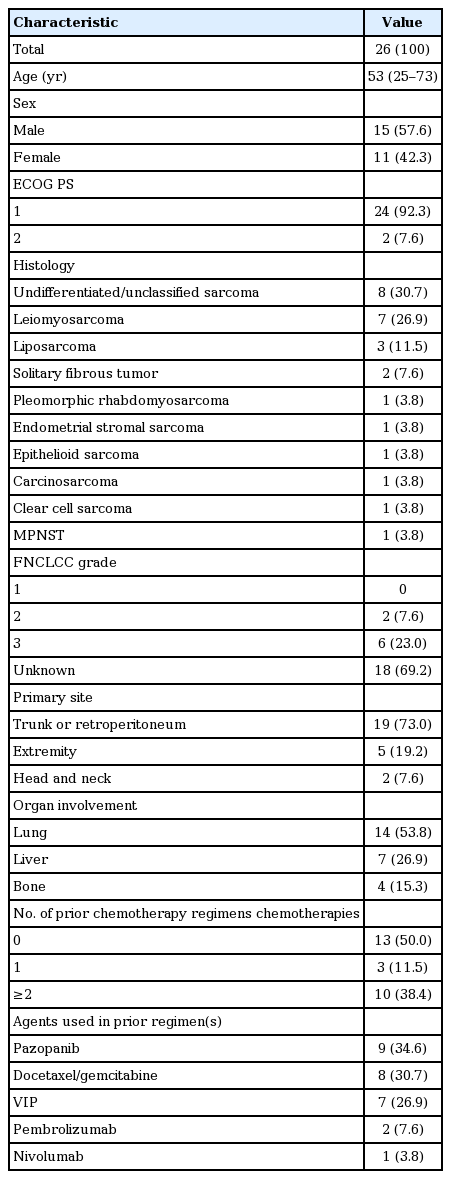
Notably, a 45-year-old male patient with dedifferentiated liposarcoma from the retroperitoneum had rapidly progressive disease. He underwent prior chemotherapy including adriamycin and cisplatin, docetaxel and gemcitabine, pazopanib, paclitaxel, etoposide and cisplatin, doxorubicin, ifosfamide, and dacarbazine, and pembrolizumab. However, he was alive for 7 years at the time of this writing owing to olaratumab and doxorubicin with partial response. At the time of olaratumab/doxorubicin chemotherapy, the patient had disseminated lung metastases with extensive pleural seeding. However, after three cycles of olaratumab/doxorubicin, the patient achieved partial response and continues to respond to olaratumab/doxorubicin for 5 months at the time of this writing (Fig. 1). The cumulative dose of doxorubicin was 355 mg and there was no doxorubicin-related cardiotoxicity.
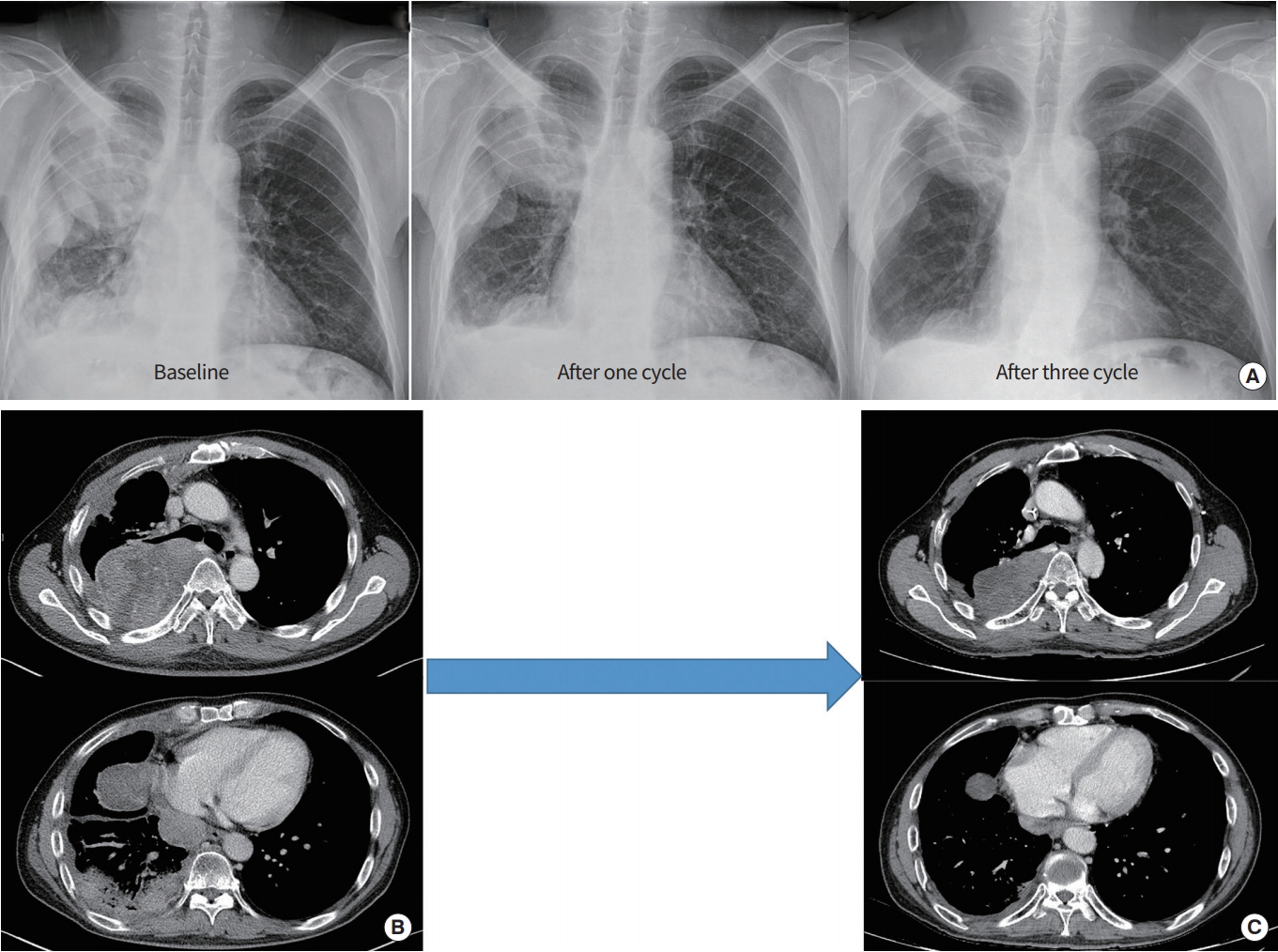
One case of partial response. (A) Forty-five-year-old male patient with dedifferentiated liposarcoma originated from retroperitoneum had disseminated lung metastasis with extensive pleural seeding at baseline. (B) After one cycle of doxorubicin, olaratumab therapy, multiple pleural masses decreased in chest X-ray. (C) After three cycles of therapy, he achieved partial response and continues to respond to olaratumab/doxorubicin for 5 months.
Survival outcomes
With a median follow-up of 11.1 months (95% CI, 11.1 to 13.1 months), the median PFS was 4.1 months (95% CI, 2.1 to 6.1 months), and the median OS was not reached (Fig. 2). We analyzed the PFS and OS in patients according to different histologic subtypes (Table 2). In patients with leiomyosarcoma, the median PFS was 4.0 months (95% CI, 1.3 to not available months), and the median OS was not reached. Meanwhile, the median PFS in patients with undifferentiated/unclassified sarcoma was 2.0 months (95% CI, 1.7 to not available). Each patient’s treatment response and survival was shown in Fig. 3.
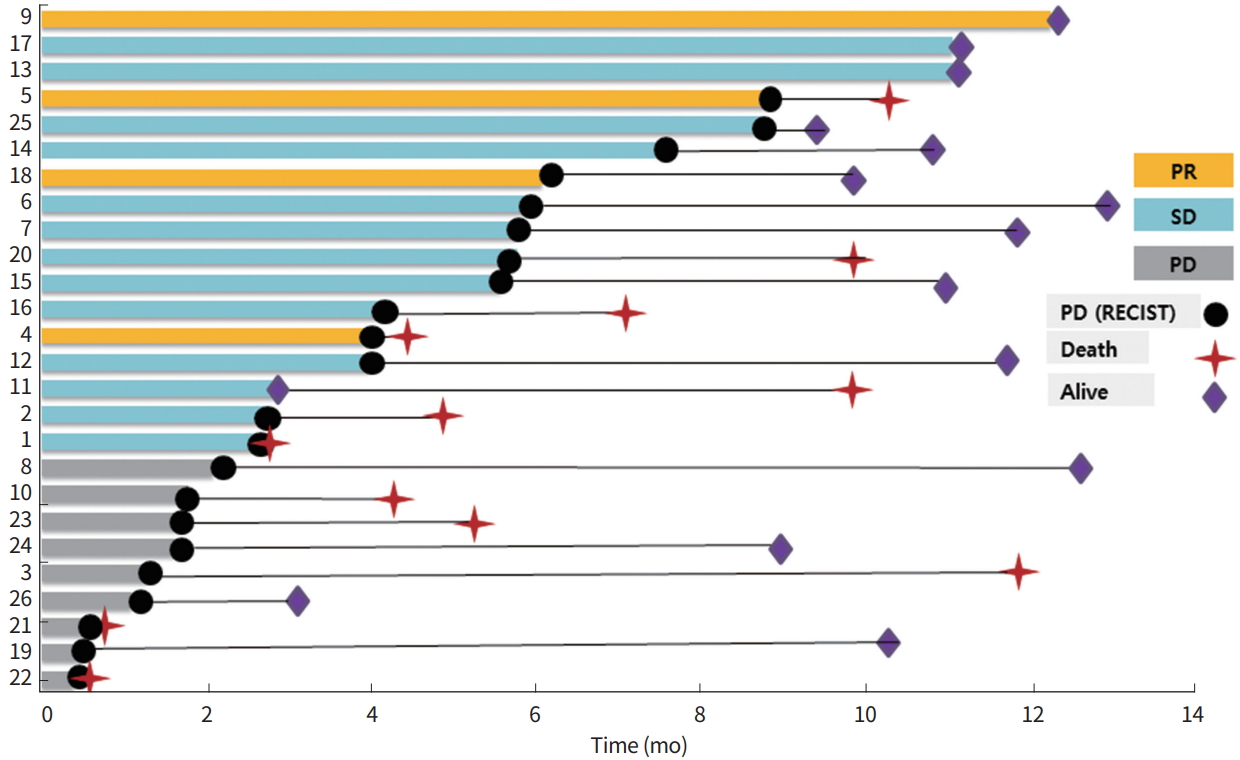
Swimmer’s plot of each patient. PR, partial response; SD, stable disease; PD, progressive disease; RECIST, response evaluation criteria in solid tumors.
Age ( > 50 years vs. ≤50 years, P = 0.089), sex (male vs. female, P = 0.169), and number of prior chemotherapy regimens (> 2 vs. ≤1, P= 0.098) were not significantly correlated with PFS at univariate level. However, performance status (ECOG 2 vs. 1) was a significant adverse factor of PFS for olaratumab/doxorubicin treatment (P= 0.001).
Treatment-related toxicity
As shown in Table 3, the most common non-hematologic toxicities were anorexia (grade 1–2, n= 6), nausea (grade 1–2, n = 3), mucositis (grade 2, n = 2), fatigue (grade 1–2, n = 3), constipation (grade 1, n= 1), diarrhea (grade 2, n= 1), and skin rash (grade 1, n= 1). Grade 3 hematologic toxicities were neutropenia (n = 7), febrile neutropenia (n = 4), and anemia (n= 1). There was no treatment-related mortality associated with olaratumab and doxorubicin treatment both in frontline or salvage settings. Most non-hematologic toxicities were reversible with appropriate medical management (e.g., loperamide for diarrhea) and dose reduction. The dose of olaratumab was reduced in 15.3% of patients (n = 4) due to treatment-related toxicities.
DISCUSSION
We demonstrated that olaratumab and doxorubicin are a feasible treatment option with acceptable anti-tumor activity in metastatic STS patients. Moreover, the regimen was relatively safe and efficacious in heavily pre-treated patients with metastatic sarcoma. Of note, we observed that olaratumab and doxorubicin tended to have better anti-tumor activity in patients with leiomyosarcoma, but less activity in patients with undifferentiated/unclassified sarcoma.
Two open-label dose-escalation phase I studies evaluated olaratumab as a single agent in patients with advanced solid tumors [10]. The combination of olaratumab plus doxorubicin has provided a new front-line therapeutic option for STS patients. An open-label phase Ib and randomized phase II trial of patients with advanced STS demonstrated that the addition of olaratumab to doxorubicin prolonged PFS by 2.5 months and OS by 11.8 months than doxorubicin alone [11]. The recently reported results of A Study of Doxorubicin Plus Olaratumab (LY3012207) in Participants With Advanced or Metastatic Soft Tissue Sarcoma (ANNOUNCE), the phase III study of olaratumab in combination with doxorubicin in patients with advanced or metastatic soft-tissue sarcoma, did not confirm the clinical benefit of olaratumab in combination with doxorubicin as compared to doxorubicin, a standard-of-care treatment [12]. Continued approval depend on the verification of clinical benefits in a confirmation trial.
In terms of pediatric-type sarcoma, olaratumab, alone and in combination with standard of care, blocks the growth of some preclinical PDGFRα-expressing pediatric bone and soft tissue tumor models [13]. Therapeutic targeting of PDGFRα with small-molecule inhibitors, such as sunitinib and imatinib, has been investigated preclinically and clinically in multiple pediatric cancer types, including osteosarcoma and malignant rhabdoid tumors [14]. Although PDGFRα is not frequently altered in childhood cancers [15], receptor expression is associated with progressive disease and poor prognosis in some pediatric malignancies of bone and soft tissue [16]. Moreover, although PDGFRα expression in cell lines and xenograft models was necessary for olaratumab to demonstrate some antitumor activity in preclinical models, expression alone was insufficient to predict sensitivity [2].
Regarding toxicity profile, despite the retrospective nature of our study, we used the same dosage and dose modification with the phase Ib/II clinical trial (patients received olaratumab [15 mg/kg] on day 1 and day 8 plus doxorubicin [75 mg/m2] on day 1 of a 21-day cycles). In our study, toxicities were less frequent than those in the phase Ib/II clinical trial.
Some limitations of this study include its retrospective nature and small sample size, particularly for histologic subgroup analyses. However, to the best of our knowledge, this is the first study to date to report the efficacy of olaratumab and doxorubicin specifically in an Asian population. In summary, olaratumab plus doxorubicin was safe and effective for patients with advanced STS and should thus be considered as a treatment option for STS patients who have a suitable performance status and adequate heart function.
Notes
No potential conflict of interestrelevantto this article was reported.
Acknowledgements
This work was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI14C2750, HI14C3418).