Contrast-induced encephalopathy and nonconvulsive status epilepticus after diagnostic cerebral angiography in an end-stage renal disease patient
Article information
Abstract
A 70-year-old man with a history of recurrent ischemic stroke and end-stage renal disease was admitted to the neurology department for a transient ischemic attack. The patient underwent transfemoral cerebral angiography with iopamidol to evaluate the status of carotid stenosis. On the same day, the patient developed drowsy mentality, global aphasia, and fever. Electroencephalography showed continuous regional rhythmic delta activities (0.5 to 1.0 Hz) without definite spatiotemporal evolution, suggestive of focal seizure disorder arising from the left temporal area and ictal-interictal continuum. Computed tomography perfusion images showed hyperperfusion in the left hemisphere. The patient was diagnosed with contrast-induced encephalopathy and associated nonconvulsive status epilepticus. The patient was treated with oral lacosamide, levetiracetam, and daily hemodialysis. The patient’s mental status recovered after 8 days of intensive care unit care.
INTRODUCTION
While cerebral angiography is routinely performed, it is associated with a small but definite risk of neurological complications. Transient neurologic events are the most common (0.34%) and are usually attributable to embolization due to stagnant blood or disruption of carotid plaques [1]. However, in some cases, unexpected rare complications can manifest. Contrast-induced encephalopathy (CIE) is a rare complication that usually manifests as cortical blindness, aphasia, and altered mental status, which are attributable to the intra-arterial administration of iodinated contrast medium. Here, we report a case of CIE with nonconvulsive status epilepticus (NCSE) after angiographic evaluation of carotid stenosis in an end-stage renal disease (ESRD) patient.
CASE REPORT
A 70-year-old male presented with transient left-sided weakness and dysarthria. The patient was admitted to the neurology department with a diagnosis of transient ischemic attack. The patient had a history of diabetes, hypertension, and three previous ischemic strokes. He was started on hemodialysis for ESRD 5 years ago. Initial brain imaging findings did not show acute lesions or perfusion deficits in the diffusion weighted-magnetic resonance imaging (MRI) images. In the computed tomography (CT) perfusion images (Fig. 1A), there was significant bilateral stenosis of the carotid arteries, but there was no perfusion deficit. After admission, the patient was asymptomatic, but due to the recurrent ischemic stroke history, a diagnostic cerebral angiography was planned to evaluate whether carotid stenting was needed.
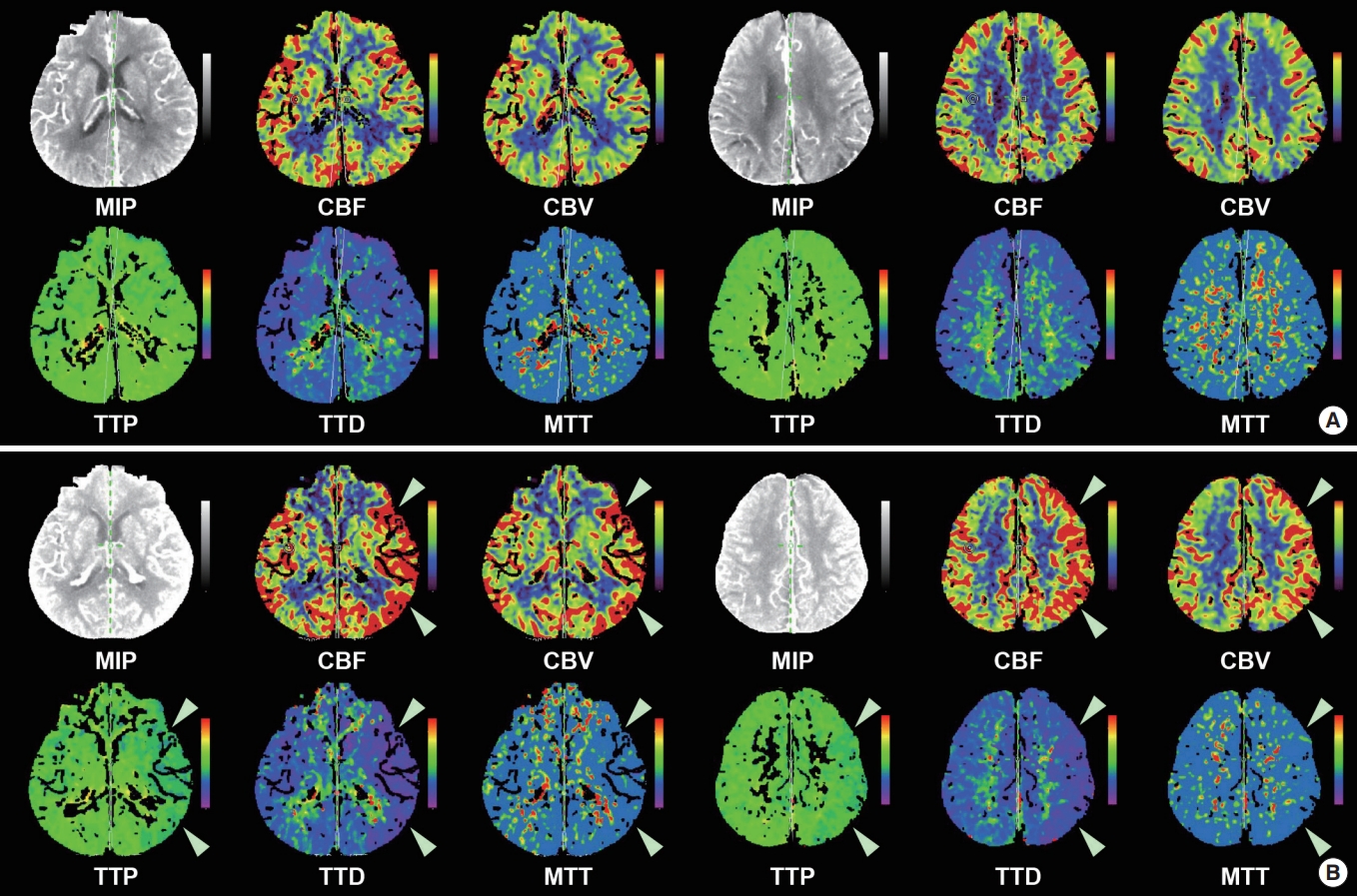
Computed tomography perfusion images of the patient. (A) The perfusion images taken at the time of presentation to the emergency department do not show any perfusion deficits. (B) However, hyperperfusion of the left hemisphere (white arrowheads) is noted in the perfusion images taken when the patient developed aphasia and altered mental status after cerebral angiography. MIP, maximal intensity projection; CBF, cerebral blood flow; CBV, cerebral blood volume; TTP, time to perfusion; TTD, time to drain; MTT, mean transit time.
On the third hospital day, a diagnostic cerebral angiogram was performed at 10:30 AM (Fig. 2) with Scanlux (Iopamidol, nonionic, low-osmolar iodinated contrast agent; Sanochemia Pharmazeutika, Wien, Austria), and a total of 120 mL was used. During and after the procedure, the patient had a high blood pressure of 209/82 mm Hg, but since he was an ESRD patient with high basal blood pressure, no meticulous blood pressure control was performed. After the procedure, the patient did not show any neurological deficits, though he felt slightly nauseated. The patient underwent hemodialysis on the afternoon of the same day for 3.5 hours. Six hours after the cerebral angiogram, during hemodialysis, the patient gradually became drowsy and could not answer questions appropriately. The patient concurrently developed a high fever of 38.8°C that evening, which could not be controlled by antipyretics. An emergent CT scan of the brain did not reveal any new lesions. The patient’s mental status did not recover the next day, and while he was nearly alert, he was globally aphasic. There was mild weakness of the right upper and lower extremities with an upgoing Babinski sign. The patient was transferred to the stroke unit, and electroencephalography (EEG), CT perfusion angiography, and brain MRI were performed.
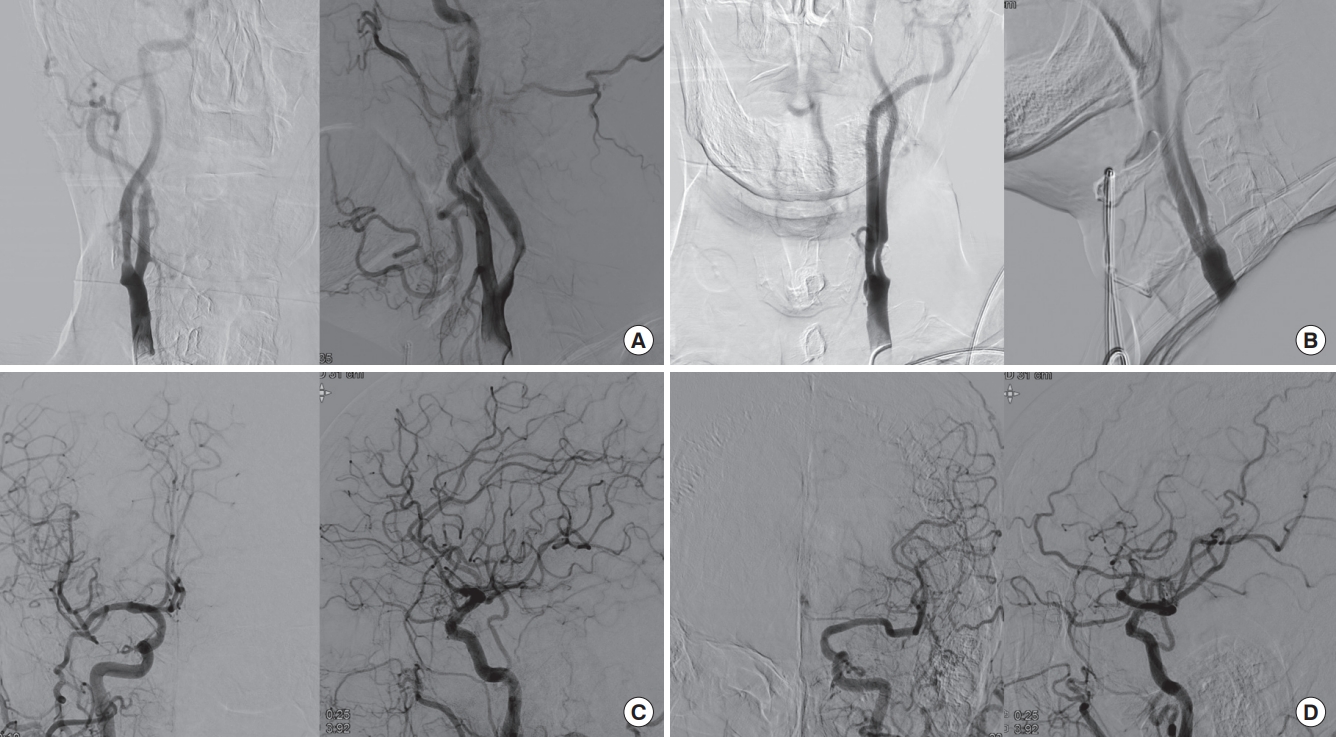
Transfemoral cerebral angiography findings of the patient. The (A) right and (B) left carotid angiography show about 50% and 70% stenosis. The (C) right and (D) left intracranial angiography do not show any arterial occlusion or filling defects.
The EEG showed continuous moderate amplitude regional rhythmic delta activities (0.5 to 1 Hz) without definite spatiotemporal evolution in the left temporal area, suggestive of focal seizure disorder arising from the left temporal area and compatible with ictal-interictal continuum (Fig. 3). The CT perfusion images showed hyperperfusion in the left hemisphere (Fig. 1B), which was compatible with the ongoing ictal activity. Intravenous (IV) lacosamide 200 mg was administered to the patient, and oral lacosamide 100 mg Bid and levetiracetam 500 mg Bid were maintained. Brain MRI obtained 35 hours after symptom onset showed several tiny acute infarctions in the bilateral parieto-occipital area, though these were not considered to be responsible for the patient’s mental status (Fig. 4). The next day, the patient’s mental status changed to deep drowsy, but the aphagia improved, and he could obey simple commands. Under the impression of CIE and associated NCSE, oral antiepileptic drugs were maintained, and daily hemodialysis was administered. A strict blood pressure control was also performed with IV nicardipine due to concerns of posterior reversible encephalopathy syndrome. The patient’s mental status and mild right-sided weakness gradually improved, and after 8 days of neurocritical care, the patient became alert, with some mild residual confusion. The patient was discharged 16 days after when the cerebral angiography was performed.

Electroencephalography (EEG) of the patient. (A) EEG taken after the onset of aphasia and altered mental status shows continuous moderate amplitude regional rhythmic delta activities (0.5 to 1 Hz) without definite spatiotemporal evolution in the left temporal area. (B) EEG taken after the intravenous administration of antiepileptic drugs showing improvements, with clinical correlation.

Brain magnetic resonance imaging diffusion weighted images. Tiny diffusion restriction is shown in the bilateral parieto-occipital area, though this was not considered to be responsible for the patient’s mental status.
Written informed consent by the patients was waived due to a retrospective nature of our study.
DISCUSSION
CIE is an acute, generally reversible, neurological disturbance attributable to the intra-arterial administration of iodinated contrast medium [2]. While transient cortical blindness is the most commonly reported neurological syndrome, encephalopathy, motor and sensory disturbances, aphasia, and seizures have also been reported. It can be usually differentiated from embolic complications by its delayed onset after angiography [3]. Cortical or subcortical contrast enhancement on CT and vasogenic edema on magnetic MRI are typical findings [2], though these may be normal. The known risk factors for CIE include hypertension, diabetes mellitus, renal impairment [4], or injection of large volumes of iodinated contrast [2]. Patients with CIE may also develop high fever [3], contributing to the disruption of the blood-brain barrier (BBB) and increased brain microvascular permeability. While the delayed symptom onset, typical clinical course, and comorbid risk factors in this patient support the diagnosis of CIE, brain imaging lacked the typical findings of CIE, making it hard to clearly differentiate between CIE and other differential diagnoses. In addition, concurrent NCSE on EEG and hyperperfusion of the corresponding hemisphere were noted. In clinical practice, we believe that the prompt use of antiepileptic drugs, daily hemodialysis, and strict blood pressure control to cover all differential diagnosis led to improvement in the patient. In retrospective, it is likely that tiny embolic infarcts may have precipitated CIE in a high risk patient, followed by concurrent NCSE.
The novelty of this case is the comorbid occurrence of CIE and NCSE. Seizures are a frequent symptom of CIE. However, their characteristics and frequency have not been previously reported. In a recent systemic review of CIE after cardiac catheterization, seizures occurred in four of 52 cases, but confusion and change in consciousness were observed in a larger number of patients. Considering that these patients may have been managed primarily by non-neurologists, there is a chance that nonconvulsive seizures may have been missed in these patients. As such, the frequency of seizures may have been much higher. In our patient, the presence of aphasia as a subtle ictal phenomenon, focal rhythmic activity higher than 0.5 Hz, clinical response to antiepileptic drugs, and hyperperfusion of the corresponding hemisphere led us to the diagnosis of NCSE [5]. The unified terminology and classification system for NCSE most commonly accepted was reported in 2013 [5], and there is also a chance that reports of CIE before this period may have in insensitive to comorbid NCSE. As prompt treatment will hasten neurological recovery, careful evaluation of comorbid seizure activity should be performed if CIE is suspected, and prophylactic use of antiepileptic drugs may also be justified [3].
The contrast agent iopamidol is a nonionic low-osmolar contrast agent. It is classically considered that the hyperosmolality of the iodinated contrast contributes to CIE due to the osmotic shrinkage of endothelial cells and the resultant opening of tight junctions, causing entry of contrast agents to the central nervous system [6]. Ionized contrast agents are also associated with higher rates of BBB disruption in animal studies [6]. However, these are not essential requirements, as there have been reports of CIE with iodixanol, an iso-osmolar non-ionic contrast agent [7,8], and fatal CIE after use of iopamidol [9], the same agent used in our case. An elevated blood pressure, preceding transient ischemic attack event, and underlying ESRD may have also contributed to BBB disruption in this patient.
CIE is a rare complication. It is extremely rare after cardiac catheterization, and a recent systematic review identified a total of 52 reported cases between 1970 and 2017 [2]. It is reasonable to consider that rates of CIE would be higher after cerebral angiography due to a larger volume of contrast injected directly into the cerebral arteries. The rates of transient cortical blindness after vertebral arteriograms range from 0.3% to 1.0% [6], but the true incidence of CIE after cerebral angiography is unclear due to a number of differential diagnoses, such as embolic complications, hyperperfusion syndrome, or seizures, and ambiguity in its differentiation. While the use of nonionized low-osmolar contrast agents reduces the incidence of CIE, the growing application of cerebral angiograms in patients with multiple risk factors will likely result in higher rates of CIE. One example is mechanical thrombectomy since the target population already has BBB damage and has multiple risk factors. Thus, the clinical importance of CIE is rising, as evidenced by a recently reported rate of 1.7% after mechanical thrombectomy [10]. Future studies are needed to define the diagnostic criteria of CIE and identify the best management plans.
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception and design: HNK, SJL.
Acquisition, analysis, or interpretation of data: HNK, SYP, SK, TJK, WSJ, JSL, JMH, SJL.
Drafting the work or revising: HNK.
Final approval of the manuscript: HNK, SYP, SK, TJK, WSJ, JSL, JMH, SJL.
