The next generation of sinus stents for chronic rhinosinusitis: A systematic review
Article information
Abstract
Characterized by impaired mucociliary clearance with subsequent compromised microbial elimination, chronic rhinosinusitis (CRS) is known as a multifactorial disease process in which bacterial infection or colonization could play a role in the initiation or propagation of the inflammatory response. Various non-systemic, topical therapies have recently emerged as an alternative to conventional oral regimens in managing CRS. A topical sinonasal drug delivery system using a bioabsorbable stent represents one of the most attractive methods among multiple topical delivery options. This review discusses the commercially available topical drug delivery platform (sinus stents) for CRS and its shortcomings. Based on the current drawbacks of sinus stents, this article has also highlighted the future developmental perspectives to create the next generation of a sinus stent, thereby harnessing the full potential of the sinus stent as efficient topical drug delivery for recalcitrant CRS.
INTRODUCTION
Chronic rhinosinusitis (CRS) is an inflammatory disease of the paranasal sinuses that affects up to 16% of the population. It is responsible for annual costs (direct and indirect) of $22 billion, comprising 5% of annual healthcare expenditures in the United States [1,2]. CRS is often characterized by persistent or recurrent episodes of infection and inflammation and is divided into with and without nasal polyps [3]. Even if aggressive treatment is performed, 28% of CRS patients require continued therapy after 2 years, and 46% of the patients undergo surgical management [4].
Once CRS is identified, the recommended first-line treatment for CRS is a combination of several therapeutic agents, including short-term oral antibiotics, systemic corticosteroids, and nasal irrigation [5-7]. For a better treatment for this frequent chronic disease, a growing body of studies in topical therapies for CRS has gained attention. Topically administered therapeutic agents (anti-inflammatory therapies) can directly act on inflamed sinus tissues, delivering a higher concentration at the target site while avoiding systemic side effects [8]. Prior studies demonstrated that patients’ adherence to anti-inflammatory therapies resulted in favorable treatment outcomes [9]. Topically delivered steroid has been widely used as an anti-inflammatory therapy in CRS, especially immediately after functional endoscopic sinus surgery (FESS) [10].
Therefore, current treatment strategies alter the weight of CRS management toward topical therapies, which combine the benefits of the surgical approach with the therapeutic effect of standard medications [11]. However, multiple aspects of topical sinonasal drug delivery for CRS need to be addressed explicitly by future research, which may lead to novel therapeutic interventions with potential opportunities for personalized medicine. In this review, we will discuss the shortcomings of the currently available topical drug delivery platform, sinus stents for CRS and explore future directions in studying topical sinonasal drug delivery.
Respiratory diseases are diseases of the lungs and other parts of the respiratory system caused by various causative factors including microbial infection, smoking tobacco, and air pollution (e.g., radon and pollen). They include asthma, chronic obstructive pulmonary disease (COPD), asthma, pulmonary fibrosis, chronic bronchitis, and non-cystic fibrosis (CF) bronchiectasis. Respiratory diseases have become a growing health concern. For example, COPD is known as the third leading cause of death in the United State and the cost of COPD exceeds $50 billion in total annual costs [12]. Even if obviously low mortality of asthma, asthma is another chronic respiratory disease that affects about 22.6 million people in the United States and accounts for $81.9 billion of the total expenditure in 2013 [13].
RECENT INNOVATION: SINUS STENTS FOR TOPICAL THERAPIES AND LIMITATIONS
Medicines are changing, and numerous topical therapies have been advanced in recent research that has attempted to meet these demands [14-16]. Due to the chronic nature of CRS, sustained and prolonged drug delivery is necessary to achieve appropriate improvement. A stent implantable in the sinus cavity would be an excellent option to reach a targeted drug release in a controlled fashion as it can provide a therapeutic drug intranasally for an extended period. The recently introduced mometasone-eluting sinus stent (Propel, Intersect-Medtronic, Menlo Park, CA, USA) improved clinical outcomes by reducing synechiae formation and polyposis after FESS (Fig. 1) [17,18]. The propel sinus stent comprises mometasone furoate incorporated into a polymer-based biodegradable poly lactic-co-glycolic acid (PLGA). Once deployed inside the sinus cavity, the spring-like propel stent expands, props open into the cavity, and delivers the anti-inflammatory corticosteroid (mometasone 370 µg) directly to the sinus lining to minimize tissue inflammation. The same manufacturer has also commercialized a self-expanding arch-type stent (Sinuva, Intersect-Medtronic) [19]. This sinus stent holds much higher amounts of mometasone (1,350 µg). In a randomized, controlled, and blinded study with hundreds of CRS with nasal polyposis (CRSwNP) after FESS, patients suffering from recurrent sinonasal polyposis after endoscopic sinus surgery experienced symptomatic and endoscopic improvements over 6 months, proving that the targeted and localized delivery of steroids in a high dose via a sinus stent is an effective treatment for CRSwNP. A structurally smaller version of the PropelTM, the Propel-MiniTM was developed and now has been approved for endoscopic placement in the frontal sinuses, which demonstrated the patency of frontal sinus after placement (Fig. 2). An upgraded sinus stent with a unique structural feature has been launched to pursue long-term potent steroids delivery. LYR-210 (Lyra Therapeutics, Watertown, MA, USA) is an expandable tubular mesh with a repeat diamond pattern and can deliver up to 7,500 µg of mometasone over 24 months [20]. Compared to former sinus stents, this phase 2 study indicated that the placement of LYR-210 showed improved clinical outcomes in surgery-naïve CRS patients with and without nasal polyposis.
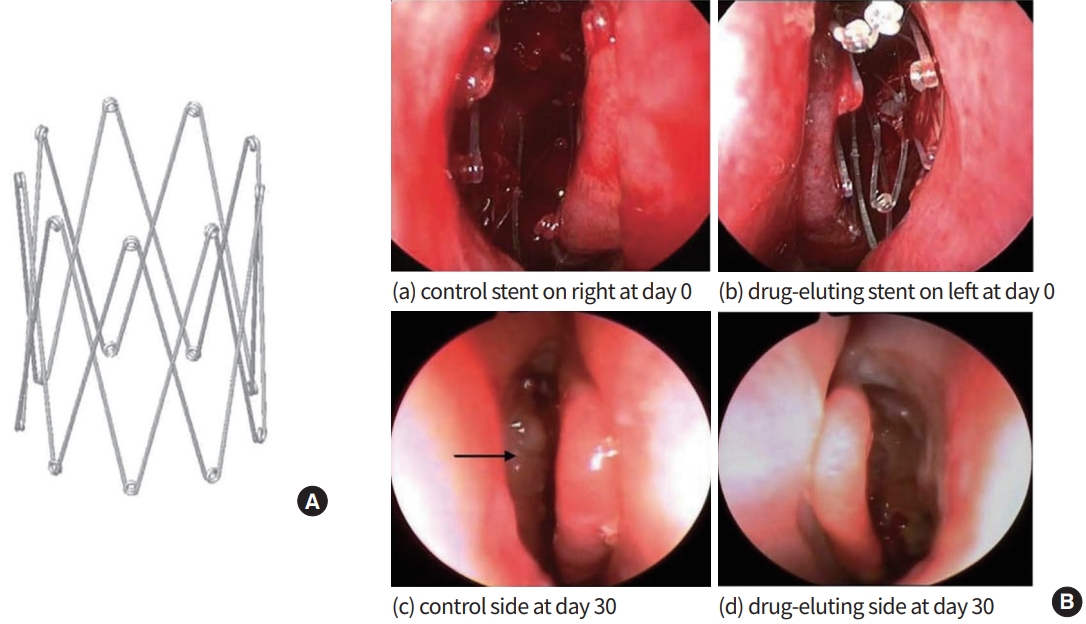
(A) The bioabsorbable drug-eluting sinus stent (Intersect-Medtronic). (B) Endoscopic photographs from a study patient. A control stent was placed in the right postoperative ethmoid sinus cavity (a), and a drug-eluting stent was placed on the left side of the ethmoid sinus cavity (b). Identical sinuses at day 30 (stents are no longer present in c and d). Middle turbinates remain in the medial position bilaterally. Polypoid mucosal change is noted in the ethmoid sinus on the control stent side (black arrow). Drug-eluting stent side (d) is normal. (a) Control stent on the right at day 0. (b) Drug-eluting stent on the left at day 0. (c) Control side at day 30. (d) Drug-eluting side at day 30. Adapted from Murr et al. [18], with permission from John Wiley and Sons.

Endoscopic findings before and after placing a Propel-Mini (Intersect®-Medtronic) bioabsorbable sinus stent. (A) Before placing a Propel-MiniTM stent in the left frontal sinus (3 months after initial frontal sinus surgery without placement of a Propel-MiniTM). (B) After placing a Propel-MiniTM stent in the left frontal sinus (3 months after revision frontal sinus surgery and sinus stent
While different stent designs were implemented to create a better mometasone-eluting sinus stent in the market, the materials used for fabricating the stents were commonly used biocompatible synthetic polymers, such as PLGA or poly L-lactic acid (PLA). Both are well-known biocompatible and biodegradable synthetic polymers approved by the U.S. Food and Drug Administration (FDA). The mechanical properties of these polymers are tunable based on the molecular weight of the polymers and the degree of crystallinity [21]. Even though the general observation of these polymers in medical devices is safe, one in vivo implantation study showed that a bar-shaped PLGA implanted under the sciatic nerve of Fisher rats over 35 days presented mild fibrosis and scarring, indicating that the degradation of the biocompatible polymers may cause tissue reactions where the devices were placed [22]. After transdermal PLA injections as a cosmetic filler onto the skin, dozens of patients subsequently developed nodules and plaques 5 to 36 months after the injection (non-necrotizing granulomatous reaction), which showed a potential foreign body reaction to human tissue [23]. Because of the acidic byproducts (i.e., lactic acid and glycolic acid) from these materials, long-term use of sinus stents in the human sinonasal epithelium may induce inflammatory responses in vivo.
In addition, a retrospective study investigating adverse events (AEs) of corticosteroid-eluting stents has been published recently based on a national database (the U.S. FDA’s Manufacturer and User Facility Device Experience [MAUDE]) [24,25]. The reported AEs were postoperative infection, periorbital cellulitis, and fungal infection [25]. Based on our unpublished in-house experience, the topical steroid has been related to fatal invasive fungal sinusitis in those patients with and without immunocompromised status.
As mentioned above, the most commercially available sinus stents are solely corticosteroid-eluting stents. No topical antibiotic delivery system is currently available even though bacterial biofilms have been implicated as a significant pathogenic factor in the recalcitrant CRS, and biofilm-forming bacteria have been shown to have increased antibiotic resistance by up to 1,000-fold, thereby diminishing the use of systemic antibiotics for CRS [26,27]. In this regard, antibiotic-eluting sinus stents promise novel therapeutic strategies for CRS.
CONSIDERATIONS IN STUDYING TOPICAL SINONASAL DRUG DELIVERY
The upper airway presents unique opportunities and chalenges when it comes to topical drug delivery. In recent years, this route has also been considered a channel for systemic molecule/drug delivery, such as the coronavirus disease 2019 (COVID-19) vaccine, inducing mucosal and systemic immune responses [28]. Bioengineers, rhinologists, pharmacologists, and other health scientists will need to understand the science behind topical sinonasal drug delivery. In developing a topical sinonasal delivery strategy, issues of absorption, distribution, metabolism, and elimination must be considered. However, addressing those local pharmacokinetics from a topical drug delivery platform is challenging as no standardized assay and formulas are available. Here are three considerations when developing a topical sinonasal drug delivery system and addressing its effectiveness.
First of all, drug-releasing patterns should be demonstrated. Controlled drug delivery applications include both sustained delivery over days/weeks/months/years and targeted delivery on the recipient tissue [29,30]. Advantages of the controlled release formulations are reducing the amount of drug necessary to provide the same therapeutic effect in patients and improving patient compliance [29]. When the topical drug delivery applications are placed in the release media and an initial large bolus of the drug is released before the release rate reaches a stable profile, it is called “burst release.” This leads to higher initial drug delivery and could reduce the effective lifespan of the application. Burst release may be an optimal delivery mechanism in several instances, such as at the beginning of treating significant infection or inflammation, which should then be followed by sustained prolonged release. Therefore, the release profile of the drug delivery application should be tailored to the pathogenesis of the disease and indication of its utilization. In an initial study using a rabbit model, a PLGA-based sinus stent coated with mometasone furoate showed a burst release pattern [31]. At 7 days post-implantation, only about 16% of mometasone was observed on the stent, indicating that most coated mometasone was released within a few days. Even though the mometasone was detected over 12 weeks through the high-performance liquid chromatography, a clinical significance after initial burst release has not been well studied. The release profiles from the commercially available steroid eluting sinus stents have not been published yet. Similar issues arise with mesh implants commonly used in orthopedic procedures to prevent postoperative infection. While drugs were released over 4 weeks, a difficult-to-control burst release was identified within the first few days of implantation, containing up to 70% of the loaded drug [32].
Secondly, the clearance mechanisms and absorption barriers of the sinonasal cavity (airway system) should be considered. When calculating the distribution and elimination of the released drug, the velocity of mucociliary transport (MCT) and volume of mucus produced in the sinonasal cavity should be taken into account. Another significant challenge for topical sinonasal drug delivery is a complex airway surface liquid (ASL, mucus) layer on the top of the sinonasal (respiratory) epithelium [33]. It is continuously produced, secreted, and finally digested, reprocessed, or discarded. Its main functions include lubrication of the epithelia, maintenance of a hydrated layer, exchange of oxygen and nutrients with the underlying epithelium, as well as acting as a barrier to pathogens and foreign substances [33,34]. CRS tissues from both CF and non-CF individuals have mucin hyperexpression and secretion, mucus accumulation, as well as submucosal gland and goblet cell hyperplasia [35]. Furthermore, the presence of impaired MCT, chronic bacterial colonization, and persistent submucosal inflammation in CRS patients resembles what is observed in CF sinus disease with thick mucus [36]. It is now well established that mucus hydration processing can be markedly compromised by environmental perturbations, such as cigarette smoke exposure, hypoxia/hypoxemia, inflammation, and infectious agents (particularly exoproducts of Pseudomonas aeruginosa) [37-40]. Therefore, in the CRS patients, the mucus layer is a barrier that can delay the transport of drugs and other molecules, and its physicochemical properties such as viscoelasticity, pH, ionic strength, and charge can impact the eventual fate and delivery of drug delivery systems in sinonasal tissue [33,41]. A better understanding of the nature of mucus and mechanisms associated with different disease states is crucial in designing efficient drug delivery systems for topical sinonasal applications.
Thirdly, potential side effects or toxicity from metabolic or degrading components should not be ignored. The safety consideration of the drug itself and the active ingredients/degrading components should be considered during the development process [42]. The human nasal mucosa has an average physiologic pH of 6.3, slightly acidic, and the maintenance of the pH in the mucus ensures the function of the ciliary clearance [42,43]. Not only the pH but also the osmolarity influences the ciliary beat and can therefore contribute to local toxicological considerations [44]. MCT and ciliary beat frequency effects are usually evaluated in the primary nasal epithelial cell model (in vitro), which may not represent the ultimate effects in vivo [42,45]. Multiple substances and compounds could be irritants to the nasal mucosa but non-damaging. Therefore, safety experiments (dose/time-dependent) in in vitro test system and a preclinical model must be evaluated carefully before concluding safety issues and initiating a phase 1 clinical trial. One appalling example of drug toxicity is the lawsuits caused by a “homeopathic” zinc-based nasal spray, Zicam (Matrixx Initiatives Inc., Phoenix, AZ, USA), in early 2000. Because this zinc-based nasal spray was classified as a homeopathic substance, it was not required to undergo stringent safety or efficacy evaluation as other conventional drugs [46]. Studies have demonstrated that intranasal administration of Zicam caused significant cytotoxicity to human nasal tissue and irreversible smell dysfunction. The health risks of intranasal Zicam use emphasized the need for stringent preclinical studies of any type of drugs or molecules to be administered topically to prevent potentially unknown and dangerous side effects in the upper respiratory system.
FUTURE DRUG DELIVERY OPTIONS
Bioengineering aspect of sinus stent
There is room for improvements with a controlled topical drug delivery for CRS. A new geometrical design of a stent would maximize the direct contact of a sinus stent with the inflamed epithelial lining of the nasal cavity. So far, few designs have been commercially adopted and launched by a few biomedical companies. An innovative design of a sinus stent should possess more flexibility but higher strength once completely deployed, thereby making full contact with the uneven sinonasal mucosal surface and supporting the sinus openings. Different materials can be utilized to improve the biocompatibility of sinus stents (tissue friendly). Although PLGA and PLA are materials for available sinus stents, polymeric composites would become a viable option for minimizing the potential immune response. For example, PLA composites containing carbon and natural fibers have been developed recently [47,48]. Reducing the polymeric portion of a stent without compromising the mechanical properties of the material would help create a more biocompatible sinus stent. A polymeric coating technique still appears to be a practical strategy to improve the extended drug profiles from a sinus stent. A novel biocompatible polymeric coating, such as phosphorylcholine (PC) polymers and polyvinyl pyrrolidone (PVP), would be potential candidates. These polymers have shown a controlled drug release in drug-eluting stents for coronary artery disease [49,50]. No acidification was also noted during the degradation process of these polymers compared to PLGA and PLA [51-54]. Furthermore, instead of directly coating the drug on the surface of a sinus stent with polymers, encapsulating drugs with liposomes or polymersomes may achieve a precise targeted prolonged delivery of desired drugs for rhinosinusitis. Liposomes are lipid capsules capable of encapsulating hydrophilic drugs with high drug-loading efficiency, while polymersomes not only can encapsulate drugs but also attach responsive molecules to stimuli (e.g., pH, temperature, and enzyme) and antibodies for targeted delivery [55,56]. Multi-layered microcapsules could be an excellent approach to creating an intelligent sinus stent. A study successfully showed that a theranostic polymer microcapsule composed of hydrogen-bonded multilayers of tannic acid (TA) and poly(N-vinylpyrrolidone) (PVPON) could gradually release low-dose anticancer doxorubicin from the multi-layered capsules under ultrasound exposure for solid tumors [57]. Based on this technology, these TA/PVPON capsules can release their encapsulated drug cargo in specific locations via externally applied ultrasound exposure where and when it is needed, “on-demand release.” Lastly, instead of coating the drug on the surface of polymeric composite, fabricating a drug-containing biodegradable polymeric scaffold (stent) might be another avenue for topical sinonasal drug delivery. For example, Jang et al. [58] recently published a delivery system using 3D printing technology: a stent embedded with paclitaxel (PTX) and an FDA-approved synthetic polymer, poly ε-caprolactone (PCL). Based on this study, the PTX within the PCL scaffold (stent) was uniformly distributed throughout, and the drug was steadily released under static and dynamic conditions in vitro.
More than corticosteroid
As noted above, bacterial biofilms have been implicated as a significant pathogenic factor in the recalcitrant CRS, and biofilm-forming bacteria have been shown to have increased antibiotic resistance [26,27]. In this regard, antibiotic-eluting sinus stents promise novel therapeutic strategies for CRS. From our group, several antibiotic-eluting sinus stents have been studied to treat P. aeruginosa to manage recalcitrant CRS. Unfortunately, multidrug-resistant P. aeruginosa is frequently found in the clinical isolates of CRS patients, leading to the failure of antibiotic treatment for recalcitrant CRS [59]. We fabricated an antibiotic-coated sinus stent (ciprofloxacincoated sinus stent). However, the surface coating of single drug (ciprofloxacin) showed a burst in vitro release profile (about 30%) within 2 days even though the sustained release of the rest of ciprofloxacin was observed for 21 days in both in vitro and in vivo. Therefore, to prevent burst release, a novel approach, dual coating, has been developed [60]. A dual-layered sinus stent was invented by incorporating two antibiotics, ciprofloxacin, and azithromycin (ciprofloxacin-azithromycin coated sinus stent [CASS]), into two different polymeric layers of a sinus stent (Fig. 3) [60]. Azithromycin, a macrolide antibiotic with hydrophobic properties, was used to create the outer coating layer of a sinus stent. At the same time, ciprofloxacin (targeting P. aeruginosa) was incorporated into the inner layer of the dual coating to prevent initial burst release from a sinus stent. Additionally, azithromycin has been known as an anti-inflammatory agent by modulating the neutrophilic activity through blockade of nuclear factor-κB and downregulation of interleukin (IL)-8, IL-6, and tumor necrosis factor-alpha inflammatory activity [61-63]. Incorporating azithromycin and ciprofloxacin in two layers demonstrated a sustained delivery of two antibiotics simultaneously over 28 days. Moreover, our in vitro observations indicate that the CASS decreased the formation and eradicated the preformed P. aeruginosa strain PAO-1 biofilms. The novel double-layered sinus stent may provide therapeutic advantages over current treatment strategies for resistant bacterial infections in CRS. Based on our in vitro results, a United States Patent has been filed and a preclinical study is underway, expecting substantial outcomes that would encourage us to plan a well-designed clinical study for proving the validity of new approach for resistant bacterial infections of CRS.
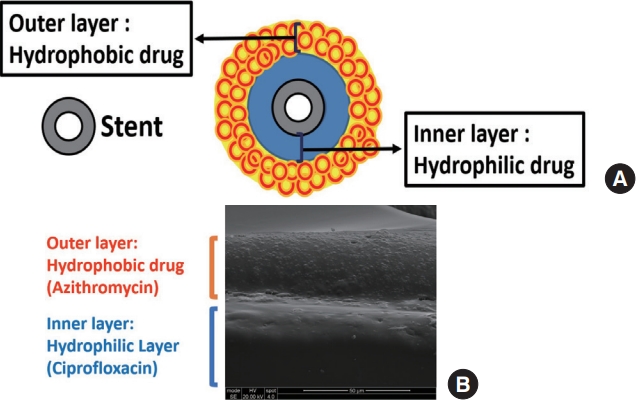
A dual-layered sinus stent. (A) Schematic illustration of a dual-layered sinus stent to deliver two drugs more efficiently (avoiding burst release). Hydrophilic drug is coated first in the inner layer, then a hydrophobic drug is covered in the outer layer. (B) Scanning electric microscope image (cross-sectional view) of the ciprofloxacin and azithromycin releasing biodegradable sinus stent. The scale bar is located at the lower right.
As another next-generation topical delivery, topical oxygen delivery has been studied in our group. Hypoxia created in the progress of CRS development has been documented as multiple aggravating factors leading to recalcitrant CRS. In the inflamed sinonasal tissues, hypoxia has been reported to cause impaired MCT, dehydration of ASL, and reduced transepithelial anion transport via the acquired chloride channel dysfunction [64,65]. Additionally, hypoxia results in pathogenic and anaerobic bacterial overgrowth, including P. aeruginosa, and elicits inflammatory response activation, which demotivates the successful rate of curing CRS even after FESS [64,66-69]. For topical oxygen delivery for CRS, we have developed a novel oxygen generating biomaterial, which is made of calcium peroxide (CPO, for oxygen generation in water), catalase (CA, immediately decomposing byproduct hydrogen peroxide [H2O2] from CPO into water and oxygen), and hydrophobic beeswax (BW, for sustained release of oxygen) [70]. The CPO-CA-BW complexes showed extended oxygen delivery over seven days with no H2O2 production from core CPO nano-aggregates, proving the potential of topical oxygen therapy as a non-antibiotic treatment strategy to improve oxygenation in hypoxic sinus tissues.
CONCLUSION
The multifactorial etiology of CRS, involving genetic factors, environmental influences, occupational factors, infection, allergy, immune dysfunction, and systemic diseases, has been recognized as the pathogenesis of CRS [71]. Topical delivery of therapeutic agents has been recognized as a viable option to treat CRS. Sinus stents have been recognized as one of the most efficient topical delivery systems, yet currently available stents have heavily focused on delivering corticosteroids to treat CRS-associated inflammation. We should understand the design, properties, release profiles, clearance mechanisms, and potential side effects when developing the next generation of topical sinonasal drug delivery. In addition, safety is critical when developing an effective drug formulation for topical drug delivery. The scale of topical sinonasal drug delivery has increased in recent years, as confirmed through increasing research studies and commercially available products from multiple biomedical companies and research institutes. However, there is room for improvements in controlled topical drug delivery, such as innovative geometric design, the biocompatibility of stent materials, coating techniques, encapsulating drugs before coating, fabricating a scaffold (stent) with drugs, and generating an on-demand delivery system “smart technology.”
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: DYC.
Acquisition, analysis, or interpretation of data: DJL, DYC.
Drafting the work or revising: DJL, DYC.
Final approval of the manuscript: DYC.
Acknowledgements
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482); National Institutes of Health (NIH)/National Institutes of Allergy and Infectious disease (K08AI146220, R21AI168894), Triological Society Career Development Award, and Cystic Fibrosis Foundation K08 Boost Award (CHO20A0-KB) to Do-Yeon Cho.
