Angiogenesis effect of udenafil in a caveolin-1 deficient moyamoya disease model: A pre-clinical animal study
Article information
Abstract
Purpose
Although pathogenic mechanisms of moyamoya disease (MMD) remain unknown, recent studies suggest that it is a caveolae disease. This study evaluated the effect of udenafil, a phosphodiesterase-5 inhibitor, on angiogenesis in in vitro and in vivo MMD models.
Methods
Angiogenesis and vessel maturation were assessed in in vitro models, caveolin- 1 (Cav-1) knockdown human umbilical vessel endothelial cells (HUVECs) and coronary artery smooth muscle cells (CASMCs), and in in vivo model of bilateral internal carotid artery occlusion (bICAo). Udenafil was administered (1,3,10, and 30 μM) in cell culture conditions, and functional studies (migration and tube formation assay) were performed and vessel maturation factors and cyclic guanosine monophosphate (cGMP) accumulation were measured.
Results
Udenafil (3 and 10 mg/kg) was orally administered once daily for 4 weeks in bICAo rat model, and histological analysis for angiogenesis and vessel maturation was performed. Udenafil increased vessel formation in both Cav-1 knockdown HUVEC and bICAo models without increased migration/proliferation of HUVECs and CASMCs. Udenafil increased CD31+ vessel density and NG2/Col4+ mural cell density in bICAo models. Cav-1 knockdown inhibited accumulation of cGMP, and udenafil treatment restored cGMP levels in Cav-1 knockdown HUVEC models. Vessel maturation factors (angiopoietin- 1 and platelet-derived growth factor receptor-β) and angiogenic factors (endothelial nitric oxide synthase) were increased after treatment with udenafil in vitro.
Conclusion
Our results indicate that udenafil reversed cellular levels of cGMP related to Cav-1 deficiency and induced angiogenesis and vessel maturation. Further studies are warranted to confirm the therapeutic effects of this strategy in MMD.
INTRODUCTION
Moyamoya disease (MMD) is an uncommon cerebrovascular disease by internal carotid artery (ICA) stenosis leading to the development of abnormal capillary at the basement of the brain [1]. Global recognition of MMD has recently increased [2]. Although the pathogenetic mechanism of MMD vessel development remain unknown, there is growing evidence that MMD is primarily a proliferative disease (vascular cell proliferation/ migration leading to luminal occlusion) characterized by aberrant angiogenesis (i.e., moyamoya vessels and thinly medial wall) [3]. We reported that caveolin-1 (Cav-1) expression were lower in MMD patients, correlating with the pathological arterial negative remodeling seen in high-resolution magnetic resonance imaging, suggesting that MMD is a caveolae disease [3,4]. In addition, lower Cav-1 expression in cellular level caused apoptosis of vascular cells and impaired angiogenesis [4].
Bypass surgery to restore cerebrovascular perfusion are the mainstay of MMD therapy, but these pose the risk of complications, such as perioperative stroke and cerebral hyper-perfusion syndrome [5]. There are no current treatments to halt progressive stenosis in MMD [5]. Medical treatment strategies for the prevention of stroke, including antiplatelet agents and statins, are of unproven benefit in MMD.
In the present study, we hypothesized that udenafil, a phosphodiesterase type-5 (PDE5) inhibitor, may be a potential therapeutic agent for increasing physiological (non-aberrant) angiogenesis in MMD models. To this end, we performed angiogenesis and migration assays, established a mechanistic basis of udenafil in in vitro experimental Cav-1 knockdown MMD models, and assessed in vivo therapeutic angiogenesis in bilateral internal carotid artery occlusion (bICAo) rat models.
METHODS
HUVEC and CASMC cultures
Human umbilical vessel endothelial cells (HUVECs), coronary artery smooth muscle cells (CASMCs), and all cell culture media (EGM-2 BulletKit and SmGm-2 BulletKit) were purchased from Lonza. HUVECs were cultured in EGM-2 complete media and CASMCs were cultured in SmGM-2 complete media. Both cell cultures were maintained at 37 ℃ in 5% CO2 conditions.
Migration assays in HUVEC and CASMC cultures
Twenty-four hours after small interfering RNA (siRNA) transfection, passage three HUVECs and passage five CASMCs were detached and re-suspended. HUVECs (1.8× 105 cells/well) and CASMCs (8× 104 cells/well) were seeded in 12 well plates and incubated at 37 ℃ in 5% CO2. After 16 hours, wounding was simulated by scratching the cell layer using a pipette tip, and the ablated cells were washed away with phosphate buffered saline (PBS). Next, cells were cultured in complete media containing udenafil (1 and 10 μM; Dong-A ST) or blank media. Cells were left to migrate toward the wounded area for 8 or 12 hours. Images for analysis were captured using bright field microscopy at 0, 8, and 12 hours. Migration fields in the images were analyzed using ImageJ software (National Institutes of Health).
HUVEC tube formation assay
Tube formation assays were performed using μ-slide angiogenesis (ibidi). In total, 10 μL of chilled Matrigel (BD Bioscience) was added to pre-cooled μ-slide wells and solidify for 1 hour at 37 ℃. Passage three HUVECs were detached using TrypLE Express Enzyme (Gibco) and re-suspended in M199 media supplemented with fetal bovine serum (1%) and heparin (5 U/mL). The udenafil concentrations selected were 1, 3, 10, 30 μM, plus a blank. Cells (1×104 cells/well) were seeded onto the Matrigel in μ-slide wells and incubated at 37 ℃ in 5% CO2. Four hours later, images were captured using a light microscope at a magnification of 40×. The number of branches and loops in the captured images were calculated using ImageJ software.
Animal surgery and udenafil treatment
Wistar-Kyoto rats were purchased from Central Lab Animal Inc. Total 47 animals were sacrificed in this study. Animals were housed in cages controlled for humidity (55%± 5%) and temperature (22± 1 ℃) with light/dark (12/12) cycles. Animals were subjected to surgery and monitored according to the guidelines of the Laboratory Animals Research Center (LARC; The Association for Assessment and Accreditation of the Laboratory Animal Care International [AAALAC]-approved facility) at the Samsung Medical Center. A 5% isoflurane was used for anesthesia induction, and it was maintained with 2% isoflurane in N2O and O2 (7:3). Homeothermic blanket system (Harvard Apparatus) was used to monitor body temperature and maintain at 37 ℃ during surgery. Rats were placed in a stereotaxic frame (KOPF). A head midline incision was made using a scalpel. The periosteum was carefully removed, and the skull was exposed. High-resolution cerebral blood flow (CBF) images were taken using the Laser Doppler Imaging system (MoorLDI2-HR, Moore Instruments Inc.). Next, the right common carotid artery, external carotid artery, ICA were exposed through a neck midline incision. The proximal and distal ICA (distance: 1 mm) was tightly double-ligated using 6–0 silk sutures, and 10 minutes later, the left ICA was occluded in the same manner. Thirty minutes later, CBF was scanned, and incisions were sutured with 5–0 monofilament sutures. Sham control groups (n=4) were operated on using only the scalp incision procedure. CBF images were acquired at 1 day after surgery. To reduce animal and surgical variation, we selected animals with a 1-day CBF of 50% or less (Supplementary Fig. 1A).
Udenafil treatment was performed following the general method reported in prior studies [6]. For oral administration, udenafil was dissolved in a Titrisol buffer (Merck). Udenafil was concentrated at 3 mg/kg/3 mL (n=10) and 10 mg/kg/3 mL (n=10) in Titrisol buffer solutions. Vehicle control (n=10) was administered as Titrisol buffer only. Udenafil was administered orally once daily for 28 days. There was no significant difference in body weight among the administration groups (Supplementary Fig. 1B).
Tissue sample preparation
Rats were perfused transcardially with 200 mL PBS, followed by 4% paraformaldehyde (PFA) in PBS under pressure control fluid delivery at 85 mm Hg using a peristaltic pump. Rat brains were post-fixed in 4% PFA for 24 hours at 4 ℃, followed by cryo-protection in 30% sucrose in PBS until they absolutely sank at 4 ℃. Coronal sections (12 μm) were taken from a 1.5 to –0.5 mm area around the bregma using a cryostat, and sections were mounted onto gelatin-coated slides and dehydrated at room temperature for 30 minutes. Slides were then stored at –20 ℃.
Immunofluorescence
For immunofluorescence staining, slide sections were first incubated in 4% PFA for 30 minutes at room temperature. To retrieve antigens, sections were incubated in sodium citrate buffer (10 mM, 0.05% tween-20, pH 6.0) at 90 ℃ for 20 minutes and cooled at room temperature for 20 minutes. Sections were then incubated in 0.25% triton X-100 in PBS for 15 minutes to enhance permeability. For blocking non-specific binding of antibodies, the sections were incubated in 10% horse serum and 0.25% triton X-100 in PBS for 40 minutes at room temperature. Sections were then incubated overnight at 4 ℃ with primary antibodies diluted in blocking solution. The following primary antibodies were used: mouse anti-CD31 conjugated Alexa fluor-488 (NOVUS Biologicals), rabbit anti-Collagen IV (Col4, Antibodies Online), and mouse anti-neuron/glia antigen 2 (NG2, Santa Cruz). After rinsing in PBS containing 0.1% tween-20 (PBST), sections were incubated with secondary antibodies for 2 hours at room temperature. The following secondary antibodies were used: Dylight 594 goat anti-rabbit immunoglobulin G (IgG, 1:200, Abcam) and Dylight 488 donkey anti-mouse IgG (1:200, Abcam). The sections were thoroughly washed in PBST three times for 10 minutes each. Finally, sections were mounted using vectashield with 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories).
Color-merged images and single-colored images were taken from immunofluorescence slides by an EVOS microscope. Each fluorescence image was analyzed in three microscopic fields by NIH ImageJ software. CD31- and Col4- immunoreactivity was measured based on fluorescence intensity from the cerebral cortex or striatum. NG2/Col4 double-positive cells were counted from the cerebral cortex.
Cav-1 siRNA transfection
SiRNA targeting Cav-1 3’untranslated region (UTR) and control siRNA were synthesized by Bioneer Corporation. The siRNA sequences were as follows: (1) Cav-1 siRNA, sense: 5’-GAGUCUGGUGAAGCUCACU-3’, antisense: 5’-AGUGAGCUUCACCAGACUC-3’; (2) control siRNA, sense: 5’-AAUUCUCCGAACGUGUCACGU-3’, antisense: 5’-ACGUGACACGUUCGGAGAAUU-3.’ siRNA transfections into HUVECs and CAMSCs were performed with lipofectamine 2000 (Invitrogen) according to manufacturer’s protocol. HUVECs at passage 3 were seeded with a density of 800,000 cells in a gelatin-coated 60-mm dish, and CASMCs at passage 5 were seeded with a density of 300,000 cells in a 60-mm dish. After 24 hours, cells were transfected with siRNA (50 nM) using lipofectamine 2000 (15 μL/dish) in Opti-MEM. Six hours later, media were changed with each complete growth medium.
Western blot analysis
Cell lysates were harvested in radioimmunoprecipitation assay (RIPA) buffer (contained with a protease inhibitor cocktail), and centrifuged at 13,200 rpm for 15 minutes. Loading buffer was added to the protein sample, denatured at 100 ℃ for 10 minutes, and then cooled at ice. Total protein samples (20 μg) were loaded on an sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel, and electrophoresis was performed. The separated proteins were transferred to a nitrocellulose membrane (pore size, 0.45 μm). Membranes were blocked with 5% non-fat milk and incubated with the primary antibody in blocking solution at 4 ℃ overnight. Next, membranes were incubated with the secondary antibody for 2 hours at room temperature. The following primary antibodies were used: mouse monoclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:5,000, Santa Cruz), mouse monoclonal Cav-1 antibody (1:1,000, Invitrogen), rabbit polyclonal endothelial nitric oxide synthase (eNOS) antibody (1:1,000, Cell Signaling), rabbit polyclonal PDBF-BB antibody (1:1,000, LifeSpan BioSciences), rabbit polyclonal angiopoietin-1 (ANGPT1) antibody (1:1,000, Abcam), and rabbit monoclonal platelet-derived growth factor receptor β (PDGFRβ) antibody (1:1,000, Cell Signaling). The following secondary antibodies were used: anti-rabbit IgG horseradish peroxidase (HRP)-linked antibody (1:1,000, Cell Signaling) and anti-mouse IgG HRP-linked antibody (1:1,000, Cell Signaling).
Cyclic guanosine monophosphate enzyme-linked immunosorbent assay
Cellular cyclic guanosine monophosphate (cGMP) levels were quantified using a cGMP enzyme-linked immunosorbent assay (ELISA) kit (Cayman Chemical). ELISA was performed according to the manufacturer’s instructions. In this study, we used S-nitrosoglutathione (GSNO) as a nitric oxide (NO) donor to increase cGMP basal levels because our preliminary ELISA study showed that cGMP was not detected in lysed cells by ELISA as basal levels were too low (data not shown). The optimal dose of GSNO was determined to be 10 μM (Supplementary Fig. 2). One day after transfection, passage four HUVECs and passage six CASMCs were seeded in individual complete media containing 10 μM GSNO (NO donor, Sigma Aldrich) in 100 mm dishes. Udenafil (final concentration: 3 or 10 μM) was added to both cells at 24 hours after seeding. After 24 hours, cells were scraped using 0.1 M hydrochloric acid. Samples were centrifuged at 1,000×g for 10 minutes. Supernatants were acetylated using 4 M potassium hydroxide and acetic anhydride. Only final samples were used in the assay. All samples were run ELISA in duplicates. Absorbance values were calculated based on the standard curve. ELISA plates were read using a SpectraMax Microplate Reader and analyzed using the SoftMax Pro Data Analysis Software (Molecular Devices).
Statistical analysis
All error bars in graphs show mean±standard error of the mean. One-way analysis of variance (ANOVA) analysis was followed by the Tukey honestly significant difference (HSD) post hoc test using SPSS version 20 software (IBM Co.).
RESULTS
Udenafil increased angiogenesis without migration/proliferation in vitro
To evaluate the role of udenafil on angiogenesis in vivo, tube formation and migration assays were performed with doses of udenafil (1 and 10 μM) under normal cell conditions. The HUVEC tube formation assay showed that 10 μM udenafil significantly increased the number of branches (F=25.879, P<0.000) and loops (F=37.640, P<0.000) compared with blank and 1 μM udenafil treatment (Fig. 1A-C). In addition, the migration assay was performed to determine whether udenafil induced migration/proliferation of HUVECs and CASMCs. Quantitative analysis showed that udenafil treatment did not change migration activity in either cell type (Fig. 1D-G).

In vitro studies for angiogenesis and migration/proliferation of human umbilical vessel endothelial cells (HUVECs) and coronary artery smooth muscle cells (CASMCs). (A) Representative images of HUVEC tube formation assay. Tube formation assay was performed to evaluate the angiogenic ability of udenafil. Images were captured at 4 hours after the assay. (B, C) Histograms of total numbers of branches and loops. Representative images of migration assay in (D) HUVECs and (E) CASMCs. Migration assay was performed to evaluate migration capability upon treatment with 1 and 10 μM udenafil. The width of the scratches was measured at 0 and 8 hours (HUVECs) or at 12 hours (CASMCs) of culture. (F, G) Histograms of relative migration values in each group. Values are presented as mean±standard error of the mean. Ctrl, control. a)P<0.001 compared to blank.
Udenafil enhanced angiogenesis with vessel maturation in vivo
To evaluate the effect of udenafil on in vivo angiogenesis, an animal study using bICAo was performed. Udenafil was daily administered orally from day 1 to 4 weeks, and animals were divided into two treatment groups based on the dose: 3 and 10 mg/kg (Fig. 2A). Angiogenesis was measured by CD31 immunofluorescence after udenafil treatment. Quantification results showed that 10 mg/kg udenafil treatment greatly increased microvessel density as compared with the vehicle control in both the cortex (F=11.156, P=0.002) and striatum (F=28.061, P=0.000) regions. Udenafil treatment at dosages over 3 mg/kg significantly increased microvessel density in the striatum (F=28.061, P=0.034) (Fig. 2B-D). Additionally, vessel maturation was measured (Fig. 2E-G). Col4 (a marker for the endothelial basement membrane) positive signaling was analyzed as indicator of vascular density (Fig. 2F) and vessel maturation was quantified by counting Col4/NG2 (a marker for pericytes) double-positive cells (Fig. 2G) in the cortex region. Col4/NG2 double-positive cells were significantly higher in the udenafil treatment group than in the vehicle group (F=11.398, P=0.006 vs. 3 mg/kg and P=0.001 vs. 10 mg/kg udenafil group).
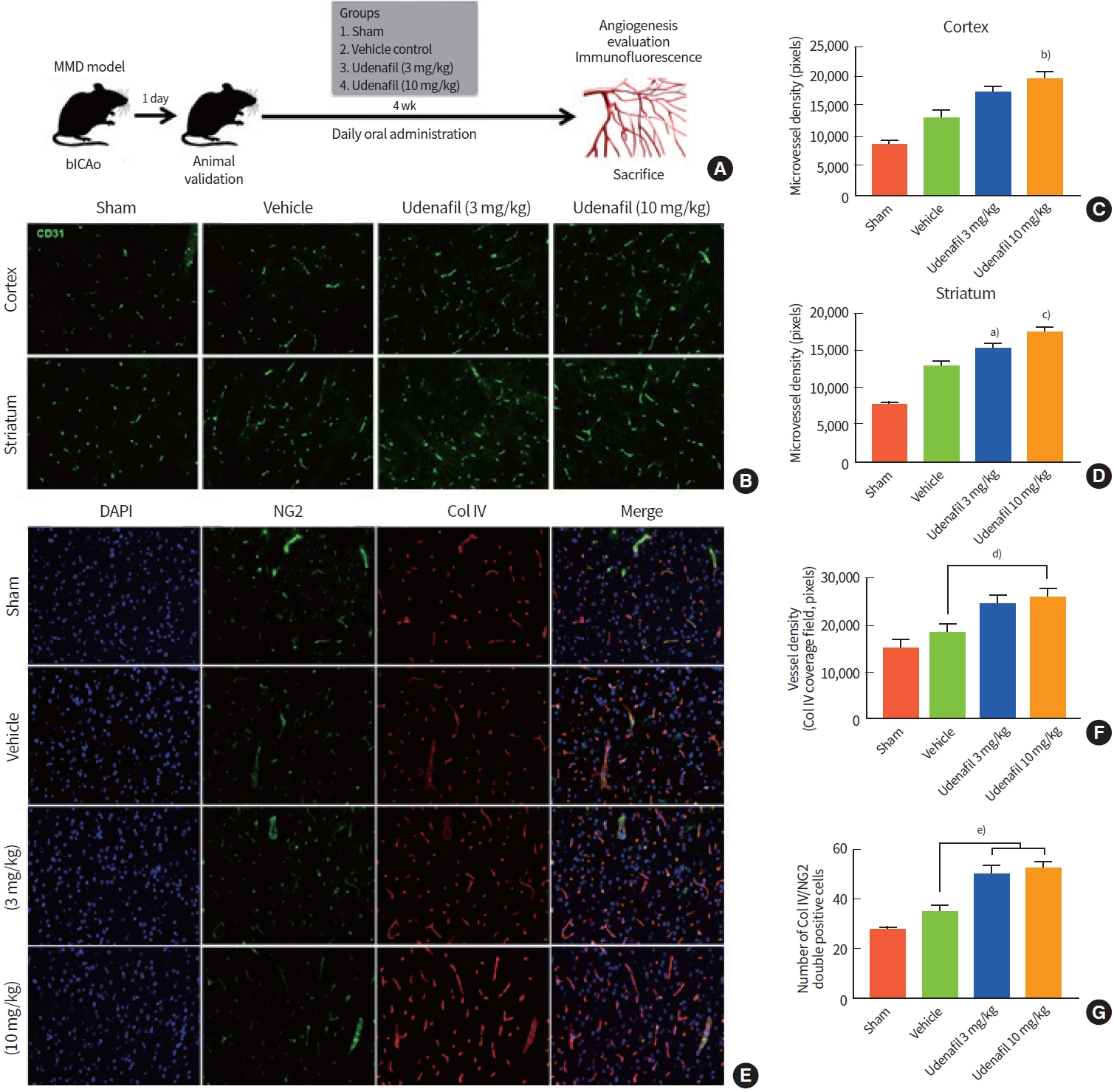
In vivo study of angiogenesis and vessel maturation in a bilateral internal carotid artery occlusion (bICAo) model. (A) Udenafil (3 and 10 mg/kg) was orally administered daily for 4 weeks after bICAo. At 4 weeks, animals were sacrificed to evaluate angiogenesis. (B) Representative images of CD31 immunofluorescence in the cortex (top row) and striatum regions (bottom row). Daily oral treatment with udenafil (10 mg/kg) after bICAo increased microvessel density in the (C) cortex and (D) striatum. (E) Representative images of double-immunofluorescence (neuron/glia antigen 2 [NG2]/4',6-diamidino-2-phenylindole [DAPI]) in the cortex region. (F) Collagen IV immunoreactive field (red fluorescence) represents endothelial basal membrane and vascular density. (G) NG2 positive cells indicate pericytes, and NG2/Col IV double-positive cells indicate mature vessels and vascular stability. Values are presented as mean±standard error of the mean. MMD, moyamoya disease. a)P< 0.05; b)P<0.01; c)P<0.001 compared to vehicle group; d)P<0.05; e)P<0.01.
Udenafil restored angiogenesis in Cav-1 knockdown in vitro models
We performed transient Cav-1 downregulation using siRNA transfection in HUVECs and performed the tube formation assay with udenafil treatment. Transfection with Cav-1 siRNA inhibited target gene expression , and udenafil did not influence levels of Cav-1 expression (Supplementary Fig. 3). In this experiment, the range of udenafil dose was increased to identify the most optimal concentration (3, 10, and 30 μM). Cav-1 siRNA (siCAV-1) transfection inhibited angiogenic ability, and udenafil treatment restored that (Fig. 3A). Quantitative analysis showed that siCav-1 transfection completely suppressed the formation of branches (F=63.327, P=0.000) and loops (F=88.198, P=0.000), and 10 and 30 μM udenafil doses significantly increased tube branches (P=0.000 vs. 10 μM and P=0.007 vs. 30 μM udenafil) (Fig. 3B) and loops (P=0.000 vs. both 10 and 30 μM udenafil) (Fig. 3C) compared with siCav-1 transfection only. Among the tested doses, 10 μM udenafil was selected as an optimal dose for further experiments.
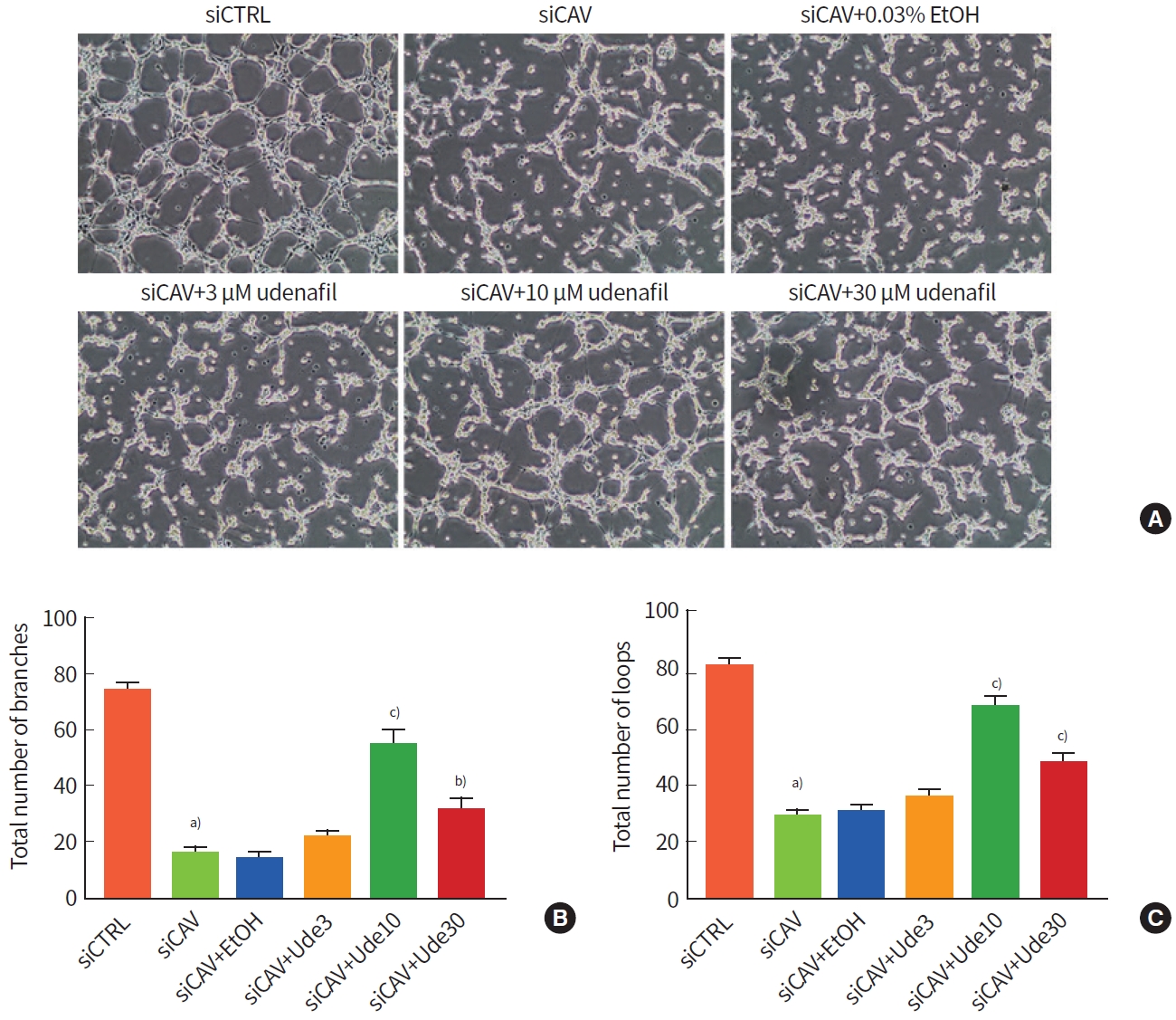
In vitro angiogenesis assay after udenafil treatment in caveolin-1 (Cav-1) knockdown human umbilical vessel endothelial cells (HUVECs). (A) Representative images of tube formation assay. Udenafil was administered in doses of 3, 10, and 30 μM. (B, C) Histograms of total numbers of branches and loops. The ethyl alcohol (EtOH) treatment group was included in this study to rule out the effect of the solvent, because EtOH was used as a solvent for the udenafil stock. Values are presented as mean±standard error of the mean. a)P<0.001 compared to control siRNA (siCTRL); b)P<0.01 and c)P<0.001 compared to Cav-1 siRNA (siCAV).
Cav-1 downregulated cGMP accumulation, and udenafil restored cGMP levels and induced expression of angiogenic and vessel maturation factors
In both Cav-1 knockdown HUVECs and CASMCs, udenafil treatment did not increase Cav-1 expression (Fig. 4A, B). However, ELISA tests for cGMP showed that Cav-1 knockdown reduced cGMP concentrations in both cell types (HUVEC, F=8.969, P=0.005; CASMC, F=4.289, P=0.032) and cGMP levels significantly increased with 10 μM udenafil treatment in Cav-1 knockdown HUVECs (P=0.048) (Fig. 4C, D). Western blot analysis was performed to analyze expression levels of eNOS and platelet-derived growth factor-BB (PDGFBB) in HUVECs and ANGPT1 and PDGFRβ in CASMCs (Fig. 4I, J). Treatment with 10 μM udenafil increased eNOS expression (F=170.376, P=0.000) in HUVECs (Fig. 4E) as well as ANGPT-1 (F=5.711, P=0.014 vs. control siRNA [siCTRL]) and PDGFRβ (F=10.403, P=0.005 vs. siCAV) in CASMCs (Fig. 4F, G). PDGFBB expression in HUVECs was significantly downregulated in siCav-1 but was not significantly increased by treatment with udenafil (Fig. 4H).

Molecular change associated with angiogenesis and vessel maturation factors after udenafil treatment. Western blot analysis was per formed to investigate the expression of angiogenic factors and vessel maturation factors in (I) human umbilical vessel endothelial cells (HUVECs) and (J) coronary artery smooth muscle cells (CASMCs). Histograms showing caveolin-1 (Cav-1) expression after small interfering RNA (siRNA) transfection and udenafil treatment in HUVECs (A) and CASMCs (B). Cycle guanosine monophosphate (cGMP) levels were measured after treatment with udenafil and S-nitrosoglutathione (GSNO) in Cav-1 knockdown HUVECs (C) and CASMCs (D). GSNO (10 μmol/L) basal media was used to upregulate baseline cGMP levels. Quantitative analysis of (E) endothelial nitric oxide synthase (eNOS), (F) angiopoietin-1 (ANGPT1), (G) platelet-derived growth factor receptor β (PDGFRβ), and (H) platelet-derived growth factor-BB (PDGFBB). (K) Possible underlying mechanism of udenafil treatment in moyamoya disease (MMD). In normal physiology, Cav-1 regulates phosphodiesterase type-5 (PDE5) in dependent homeostatic manner. In Cav-1 deficient conditions, such as MMD, Cav-1 downregulation consequently leads to cGMP accumulation by PDE5 activation, and PDE5 inhibitor treatment restores cGMP levels. Restored cGMP induces angiogenesis in endothelial cells and vessel maturation in mural cells. Values are presented as mean±standard error of the mean. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SMC, smooth muscel cell; GTP, guanosine triphosphate.a)P<0.05, b)P<0.01, and c)P<0.001 compared to control siRNA (siCTRL); d)P<0.05 compared to Cav-1 siRNA (siCAV).
DISCUSSION
The main findings of this study were as follows: (1) udenafil (10 μM) induced tube formation without migration/proliferation of endothelial cells in Cav-1 knockdown in vitro models for MMD; (2) udenafil induced angiogenesis accompanied by vessel maturation in in vivo models of bICAo; (3) angiogenic factors, such as eNOS, ANGPT1, and PDGFRβ, increased after treatment with 10 μM udenafil; and (4) Cav-1 downregulation reduced cGMP concentration, while udenafil restored cGMP levels in HUVECs.
Although PDE5 inhibitors (including udenafil, sildenafil, tadalafil, and vardenafil) have been developed for the cure for erectile dysfunction, there has recently been immense interest in finding new clinical uses of PDE5 inhibitors for various diseases, including cardiovascular disease, diabetes, and cancer [7]. PDE5 inhibitors have been found to promote angiogenesis and functional recovery after stroke [8]. Udenafil markedly attenuates the compensatory development of right ventricular hypertrophy and reduces pulmonary artery wall thickness in monocrotaline-induced pulmonary artery hypertension (PAH) models [6]. PDE5 inhibitors are now widely used in the management of PAH [9]. The treatment options established for PAH may also be effective in MMD, because MMD and PAH share many features. First, the pathological features of PAH are similar to those of MMD, such as intimal hypertrophy, arterial remodeling, in situ thrombosis, and vasoconstriction [10]. Second, with regard to biochemical features, decreased serum levels of the biomarker Cav-1 have been reported in both PAH and MMD [11,12]. In addition, it has been reported that Cav-1, a scaffolding protein of the caveolae plasma membrane, is involved in the pathogenesis of cancer and vascular diseases [13]. Studies showed that Cav-1 overexpression enhanced caveolae formation and induced capillary tube formation by nearly three-fold, while Cav-1 downregulation decreased in vitro and in vivo vessel formation and was associated with pathological angiogenesis [13-15]. Another study showed that Cav-1 involved in endotherial progenitor cell recruitment from the bone marrow [16]. Lastly, MMD and PAH share genetic similarities, namely, that homozygosity in ring finger protein 213 (RNF213) leads to MMD and PAH in the same patients or families [17,18].
Number of studies have reported vasodilating effects of PDE5 inhibitors. For example, PDE5 inhibitors have resulted in improved exercise capacity in PAH patients by increasing NO levels [19]. PDE5 inhibitors block the cGMP degradation, leading to vessel relaxation due to the increase in cGMP levels. NO stimulates cytosolic guanylate cyclase and increases cGMP levels in vascular smooth muscle cells, leading to relaxation of vascular tone [20]. PDE5 inhibitors induce vasodilation via an increase in cGMP levels in pulmonary vascular smooth muscle cells [9]. However, relatively little is known about the role of PDE5 inhibitors on angiogenesis in caveolae disease.
The present study showed that udenafil enhanced angiogenic capacity in both in vitro and in vivo experiments. Our results are in line with those of previous studies showing that PDE5 inhibitors enhance angiogenesis in endothelial cells and regulate migration and proliferation of various cell types [21-23]. And PDE5 was inhibited by direct interaction with Cav-1 [24]. The present study showed that udenafil did not affect Cav-1 expression levels in vessel cells and that Cav-1 downregulation in HUVECs and CASMCs resulted in decreased cGMP levels. Such decreased cGMP levels were restored by PDE5 inhibitors in HUVECs. These findings indicate that udenafil treatment induces the accumulation of cGMP in Cav-1 deficient conditions. Thus, udenafil could be a therapeutic modality for MMD and caveolae diseases. This is the first study to show the effects of PDE5 inhibitors in a caveolae disease model.
Our present study showed that udenafil increased vessel maturation factors and induced vessel maturation without migration/proliferation of vascular cells. Pericytes are mural cells surrounding endothelial cells in the capillary and play critical roles in vessel maturation and maintenance of vascular morphogenesis [25]. PDGFBB/PDGFRβ signaling is essential for pericyte proliferation and recruitment to endothelial progenitor cells [26,27]. A variety of signaling factors, including PDGFBB/PDGFRβ and ANGPT1/Tie-2, mediate pericyte-endothelial cell interactions, resulting in vascular maturation [28]. Aberrant angiogenesis could be associated with the development of intracranial large artery stenosis and moyamoya vessels in patients with MMD. Aberrations in pericyte-endothelial cell signaling networks contribute to tumor angiogenesis [29]. Our results of molecular analysis indicate that udenafil increases expression of vessel maturation factors in mural cells. Udenafil increased eNOS expression in endothelial cells, which is associated with angiogenesis. Likewise, udenafil upregulated PDGFRβ and ANGPT1 expression in mural cells, which are associated with pericyte-endothelial cell interactions and vessel maturation. Fig. 4K summarizes the possible mechanisms of action underlying the effects of udenafil on activation of endothelial cells and mural cells for enhanced angiogenesis with appropriate vessel maturation. Our study had limitations. In the present study, we used a bICAo model. Although bICAo is a suitable method for evaluation of therapeutic agents, this model cannot represent an MMD animal model. There is no relevant animal model for MMD, because histopathological studies of vascular wall structure in Rnf213 -/- variants showed no apparent abnormalities in mutant mice, such as intimal hyperplasia or medial layer thinness [30]. Moreover, our in vivo analysis did not contain histological data of stenotic ICA portions, since we focused on therapeutic angiogenesis from udenafil treatment in the cortex region. Further studies are needed with MMD and Cav-1 knockout animal models. We are currently developing an in vitro endothelial and smooth muscle cell system using induced pluripotent stem cells from RNF213 variant MMD patients.
The results of our study showed that udenafil reversed the decreased cellular cGMP levels associated with Cav-1 deficiency and induced angiogenesis and vessel maturation. These findings suggest that PDE5 inhibitors could be useful as a therapeutic agent to increase angiogenesis and vessel maturation in Cav-1 deficient conditions such as MMD. Notwithstanding the shortages of our disease model, our results provide a molecular background for the role of PDE5 inhibitors in MMD. Further studies are warranted to confirm the therapeutic effects of this strategy using animal models of MMD.
Supplementary materials
Cerebral blood flow (CBF) measurement and body weight record after bilateral internal carotid artery occlusion (bICAo). (A) One day CBF ratio after bICAo shows constant value among groups. (B) Body weight change for 28 days after bICAo shows no difference among groups except sham.
Cycle guanosine monophosphate (cGMP) level after S-nitrosoglutathione (GSNO) treatment. Enzyme-linked immunosorbent assay (ELISA) was performed to determine appropriate concentration of GSNO in (A) human umbilical vessel endothelial cell (HUVEC) and (B) coronary artery smooth muscle cell (CASMC).
(A, B) Western blot analysis was performed to confirm that small interfering RNA (siRNA) transfection and caveolin-1 (Cav-1) expression after udenafil treatment in human umbilical vessel endothelial cell (HUVEC). Values are mean±standard error of the mean. siCAV, Cav-1 siRNA; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. a)P<0.05 compared to control siRNA (siCTRL).
Notes
No potential conflict of interest, including CellnLife Inc., relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: DHK, OYB.
Drafting the work or revising: DHK, EHK, GJM, OHB.
Final approval of the manuscript: OYB.
Acknowledgements
This research was supported by a grant from the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (2018M3A9H1023675).
