Added values of transrectal ultrasonography to magnetic resonance imaging in characterizing prostate cancer: A narrative review
Article information
Abstract
Many radiologists and urologists believe that magnetic resonance imaging (MRI) is superior to transrectal ultrasonography (TRUS) in the detection and assessment of prostate cancer. Thus, they relied on MRI alone for tumor characterization. However, the role of TRUS in prostate cancer assessment remains unclear. This information on TRUS findings can aid radiologists and urologists in detecting, targeting, and staging cancers. Additionally, there are some imaging features that are unseen on MRI, but are visible on TRUS. However, few studies have addressed the added value of TRUS in the preoperative evaluation of prostate cancer. This review aimed to assess the added value of TRUS to preoperative MRI for prostate cancer detection, characterization, and staging.
INTRODUCTION
Magnetic resonance imaging (MRI) is a precise modality for detecting and staging prostate cancer. Therefore, MRI has become increasingly available for men with elevated prostate-specific antigen levels to detect prostate cancer based on the Prostate Imaging and Reporting and Data System (PI-RADS). Currently, PI-RADS version 2.1 has been released to improve the risk stratification of prostate cancer [1-3]. Accordingly, radiologists and urologists need to learn MRI features to understand the PI-RADS. However, focal lesions are still categorized based on MRI findings alone. Besides, there is a wide variation in PI-RADS categorization in terms of interpretation experience, education, and MRI quality [4-7].
Recently, high-resolution transrectal ultrasonography (TRUS) has the potential to provide more detailed imaging features of prostate cancer [8-10]. Some imaging features are not visible on MRI, but are visible on TRUS alone [8-10]. TRUS cannot replace MRI completely in characterizing prostate cancer, but there is an increasing need for radiologists and urologists to use TRUS for cancer detection and biopsy. To the best of our knowledge, only a few reports have shown the added value of TRUS in the preoperative staging of prostate cancer. However, MRI has some limitations in the preoperative staging work-up or the differentiation of insignificant and significant prostate cancers. This review aimed to assess the added value of TRUS to preoperative MRI for cancer detection, characterization, and staging.
TUMOR LOCATION: MRI VS. TRUS
Before localizing a lesion on TRUS, we need to know the difference between MRI and TRUS in terms of lesion location. An MRI axis is usually perpendicular to the prostate urethra, whereas that of TRUS is oblique, coronal to the prostate [11]. Therefore, when a lesion is closer to the posterior capsule on MRI, it is detected more supperiorly on TRUS (Fig. 1A) [12,13]. Radiologist and urologist can be surprised to see a lesion in the base although it is located around the posterior capsule of mid-gland [14,15]. In contrast, when a lesion is closer to the anterior capsule on MRI, it is detected more inferiorly on TRUS (Fig. 1B) [12,13]. On TRUS, they should find a lesion at the apex when it is near the anterior capsule of mid-gland on MRI [14,15]. Also, the lesion size and shape can differ between MRI and TRUS. It is not surprising to see that a tumor size was greater or less than 1.5 cm on TRUS, even though it was a PIRADS 4 or 5 tumor. From this point of view, radiologists and urologists need to be familiar with the difference in lesion location between MRI and TRUS to precisely detect it. This is the first step in local tumor staging using TRUS. Unless radiologists and urologists understand this difference, they cannot detect or assess a focal lesion on TRUS, even though they are categorized as PI-RADS 4 or 5 on MRI [11-13,16,17]. For the same reason, the size and shape of a lesion is different between MRI and TRUS.
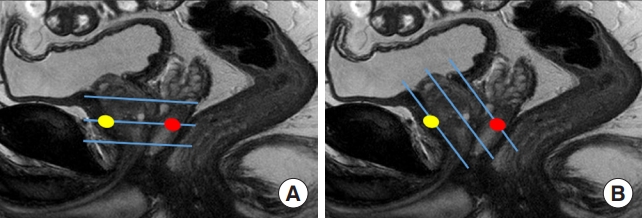
Schematic figures comparing the scan axes of magnetic resonance imaging (MRI) and transrectal ultrasonography (TRUS). (A) Three transverse blue lines represent the scan axis, which is perpendicular to the prostate urethra on MRI. One anterior (yellow) and one posterior (red) lesions are placed on the middle line. (B) Three oblique blue lines represent the scan axis, which is oblique to the prostate urethra on TRUS. The anterior lesion (yellow) is placed onto the lower line, whereas the posterior lesion (red) is placed onto the upper line.
PERIPHERAL CANCER: SIGNIFICANT VS. INSIGNIFICANT
Generally, peripheral cancer appears as a hypointense tumor on T2-weighted imaging (T2WI) and hyperintense tumor on diffusion-weighted imaging (DWI). As the Gleason score (GS) increases, a lesion tends to be more hypointense on T2WI and more hyperintense on DWI (Table 1). Tumor size is useful only for differentiating between PI-RADS 4 and 5. On TRUS, peripheral cancer is usually observed as a hypoechoic tumor. As the GS increases, a lesion tends to be more hypoechoic (Table 1). Additionally, the contour of a peripheral cancer becomes irregular and infiltrative when it becomes significant (Table 1). These morphological changes are well observed on TRUS, but are not clearly defined on MRI (Figs. 2, 3). Therefore, PIRADS version 2.1 does not use these findings in lesion categorization [18]. All anatomical changes, except tumor size, do not contribute to differentiating PI-RADS 4 and 5 peripheral lesions, although the frequency of significant cancers is significantly different [18,19]. This is one of the limitations of the PI-RADS version 2.1 decision rules.
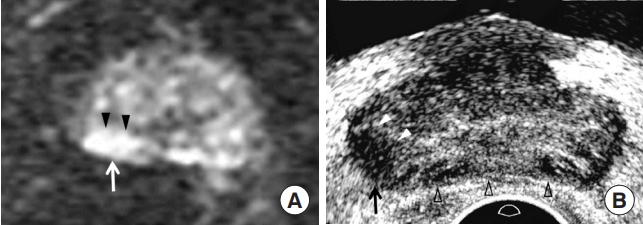
Prostate Imaging and Reporting and Data System (PI-RADS) 4 peripheral cancer in a 78-year-old man. (A) Diffusion-weighted imaging showing a 1.1 cm hyperintense peripheral lesion (white arrow) in the right mid-gland, consistent with PI-RADS 4. The shape of the lesion is lenticular, with a smooth contour (arrowheads). His prostate-specific antigen was 4.24 ng/mL. (B) Transrectal ultrasonography (TRUS) showing the tumor (black arrow) located at the right base measuring 0.8 cm. The tumor shape is oval with irregular contours (white arrowheads). Prostate compression (black blank arrowheads) was minimized using the transducer to depict the posterior lesion, as much as possible. The tumor-to-tissue contrast is significant because TRUS uses fundamental imaging and a low dynamic range. Histological diagnosis was Gleason score 7 (4+3) adenocarcinoma.
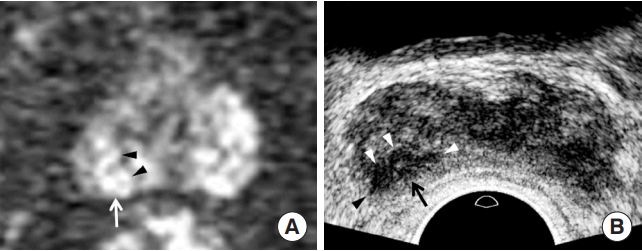
Prostate Imaging and Reporting and Data System (PI-RADS) 5 peripheral cancer in a 71-year-old man. (A) Diffusion-weighted imaging (DWI) shows a 1.3 cm hyperintense peripheral lesion (white arrow) in the right base, consistent with PI-RADS 4. The tumor shape is oval, with a smooth contour (black arrowheads). No extracapsular extension was observed. His prostate-specific antigen was 6.62 ng/mL. (B) Transrectal ultrasonography (TRUS) showing that the tumor is a 1.1 cm hypoechoic peripheral lesion in the higher base compared to that on DWI. The tumor shape is lenticular, with an irregular contour (white arrowheads). The black arrowhead indicates extracapsular extension. The tumor-to-tissue contrast is significant because TRUS uses fundamental imaging and a low dynamic range. The histological diagnosis was Gleason score 7 (4+3) adenocarcinoma.
TRANSITION CANCER: SIGNIFICANT VS. INSIGNIFICANT
Generally, the charcoal-eraser sign is a well-known T2WI feature [20]; however, it requires time and experience to apply it in daily practice. Therefore, the current PI-RADS version 2.1 describes the charcoal-eraser sign as a lenticular or non-circumscribed homogeneous mass with moderately hypointense signal intensity on T2WI [1,21]. However, it does not state which MRI features are suggestive of significant cancer except for increasing tumor size (Table 1). In contrast, transition cancers become more heterogeneous and hyperechoic, in addition to increasing tumor size as the GS increases (Table 1, Figs. 4, 5) [12,22]. Additionally, the contour of a transition cancer becomes irregular and infiltrative as the GS increases [22]. Similar to peripheral cancer, all anatomical changes except tumor size do not contribute to differentiating between PI-RADS 4 and 5 transition lesions, although the frequency of significant cancers is significantly different [18,19]. This is one of the limitations of the PI-RADS version 2.1 decision rules.
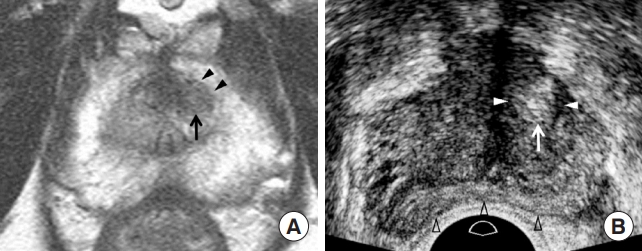
Prostate Imaging and Reporting and Data System (PI-RADS) 4 transition cancer in a 74-year-old man. (A) T2-weighted imaging showing a non-circumscribed homogeneous transition lesion (black arrow) in the left anterior mid-gland. The lesion is a 0.8 cm moderately hypointense tumor. The shape and contour of the lesion are oval and smooth, respectively (black arrowheads). His prostate-specific antigen was 3.07 ng/mL. (B) Transrectal ultrasonography (TRUS) showing a tumor located in the inferior mid-gland. The tumor is a 0.9 cm homogeneous hyperechoic mass (white arrow), with a hypoechoic rim (white arrowheads). The shape and contour of the lesion are oval and smooth (white arrowheads), respectively. The prostate (black blank arrowheads) is compressed, as much as possible, by the transducer to depict an anterior lesion. The tumor-to-tissue contrast is significant because TRUS uses fundamental imaging and a low dynamic range. Histological diagnosis was Gleason score 7 (3+4) adenocarcinoma.
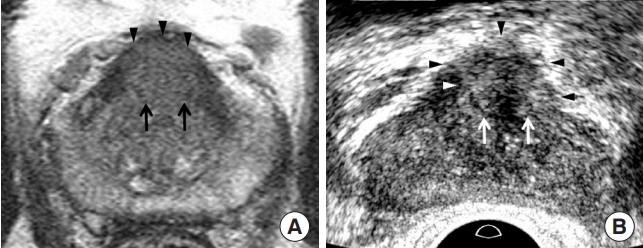
Prostate Imaging and Reporting and Data System (PI-RADS) 5 transition cancer in a 62-year-old man. (A) T2-weighted imaging showing a 1.6 cm poorly-circumscribed homogeneous transition mass (black arrows) in the anterior midline base. The lesion was moderately hypointense. The tumor shape and contour are oval and smooth, respectively (black arrowheads). His prostate-specific antigen was 9.10 ng/mL. (B) Transrectal ultrasonography (TRUS) showing that the tumor was located in the anterior midline of the mid-gland. The tumor is a 1.7 cm hyperechoic tumor (white arrows), with a hypoechoic rim (white arrowhead). The lesion shape and contour were oval and irregular (black arrowheads), respectively. The tumor-to-tissue contrast is significant because TRUS uses fundamental imaging and a low dynamic range. The histological diagnosis was Gleason score 7 (4+3) adenocarcinoma.
LOCAL AGGRESSIVE FEATURES
Extracapsular extension (ECE) and seminal vesicle invasion(SVI) are typical locally aggressive features of prostate cancer. MRI is a good imaging modality for depicting ECE [23-25]. However, TRUS can provide additional findings to determine ECE when MRI is inconclusive [13,18]. A thick and irregular surface of a prostate capsule is a good finding suggesting ECE on TRUS (Fig. 3). Color Doppler TRUS helps to enhance the chances of identifying ECE if it shows hypervascular blood flow crossing the capsule from the tumor to the extracapsular fat. It is not difficult to detect ECE using TRUS if radiologists or urologists know how to achieve good image quality. MRI has been reported to be useful [26-28]; however, the role of TRUS in depicting SVI is controversial. It can show thick hypervascular walls in the seminal vesicle. Further investigations are necessary to demonstrate how TRUS detects SVI.
CURRENT LIMITATIONS OF USING TRUS
Many radiologists and urologists perform TRUS procedures; however, almost none of them can obtain a good image quality or perform biopsy techniques. They must use fundamental scanning instead of harmonic scanning, which is the default setting for current TRUS scanners. The former offers better tissue contrast than the latter to improve tumor depiction [12,13]. Another scanning technique for increasing tissue contrast is to use a lower dynamic range of < 50 [12,13]. These scanning techniques decrease image resolution, but increase tissue contrast to help differentiate prostate cancer from normal tissues (Figs. 2-5). Therefore, although fundamental scanning and low dynamic range reduce image resolution of TRUS, these protocols contribute to tumor detection and characterization. As a result, it is easy to identify a tumor that is more contrasted with normal tissue and to assess tumor size, echogenicity, texture, and contour.
When performing TRUS, radiologists and urologists should minimize prostate compression prior to tumor detection (Fig. 2). Frequently, small peripheral cancers are difficult to detect because they tend to be embedded due to transducer compression. Additionally, this compression deforms the prostate contour from a triangular to a banana shape (Fig. 4) [13]. This contour deformity also makes MRI-TRUS image fusion more difficult to register [12,13]. Therefore, a transducer should be placed in the posterior prostate capsule until a focal lesion is detected to avoid prostate deformation. The transducer is then pushed to the target tumor if the lesion contour is not too deformed. The contour deformity increases as the tumor approaches the posterior capsule. Therefore, transducer compression does not influence tumor contour so much, as the tumor approaches the anterior capsule (Figs. 4, 5).
CONCLUSION
TRUS can show various imaging findings that are not defined to characterize a focal lesion on PI-RADS version 2.1 decision rule. Therefore, when combined with MRI, TRUS contributes to the characterization of prostate cancer. If radiologists or urologists are familiar with TRUS techniques and imaging features, they can significantly improve cancer detection and preoperative staging work-up more precisely, and then make more appropriate treatment plans.
Notes
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: BKP.
Acquisition, analysis, or interpretation of data: BKP.
Drafting the work or revising: BKP.
Final approval of the manuscript: BKP.

